[English] 日本語
 Yorodumi
Yorodumi- EMDB-24233: The cryoEM structure of LbpB from N. gonorrhoeae in complex with ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24233 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
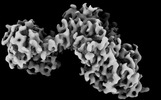
| Title | The cryoEM structure of LbpB from N. gonorrhoeae in complex with lactoferrin | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | lactoferrin / lipoprotein / neisseria / iron import / MEMBRANE PROTEIN / MEMBRANE PROTEIN-TRANSPORT PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationmembrane destabilizing activity / host-mediated suppression of viral proces / Mtb iron assimilation by chelation / phagocytic vesicle lumen / Metal sequestration by antimicrobial proteins / negative regulation of viral process / positive regulation of toll-like receptor 4 signaling pathway / negative regulation of tumor necrosis factor (ligand) superfamily member 11 production / negative regulation of single-species biofilm formation in or on host organism / positive regulation of bone mineralization involved in bone maturation ...membrane destabilizing activity / host-mediated suppression of viral proces / Mtb iron assimilation by chelation / phagocytic vesicle lumen / Metal sequestration by antimicrobial proteins / negative regulation of viral process / positive regulation of toll-like receptor 4 signaling pathway / negative regulation of tumor necrosis factor (ligand) superfamily member 11 production / negative regulation of single-species biofilm formation in or on host organism / positive regulation of bone mineralization involved in bone maturation / negative regulation of osteoclast development / antifungal humoral response / specific granule / negative regulation of lipopolysaccharide-mediated signaling pathway / positive regulation of chondrocyte proliferation / negative regulation of ATP-dependent activity / regulation of tumor necrosis factor production / bone morphogenesis / Antimicrobial peptides / negative regulation of viral genome replication / positive regulation of osteoblast proliferation / humoral immune response / Hydrolases; Acting on peptide bonds (peptidases); Serine endopeptidases / positive regulation of protein serine/threonine kinase activity / cysteine-type endopeptidase inhibitor activity / positive regulation of osteoblast differentiation / regulation of cytokine production / ossification / secretory granule / protein serine/threonine kinase activator activity / innate immune response in mucosa / cell outer membrane / iron ion transport / lipopolysaccharide binding / : / recycling endosome / specific granule lumen / antimicrobial humoral immune response mediated by antimicrobial peptide / tertiary granule lumen / antibacterial humoral response / heparin binding / defense response to Gram-negative bacterium / killing of cells of another organism / early endosome / positive regulation of canonical NF-kappaB signal transduction / iron ion binding / Amyloid fiber formation / serine-type endopeptidase activity / Neutrophil degranulation / negative regulation of apoptotic process / cell surface / protein-containing complex / proteolysis / extracellular space / DNA binding / extracellular exosome / extracellular region / nucleus / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Neisseria gonorrhoeae (bacteria) / Neisseria gonorrhoeae (bacteria) /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Yadav R / Noinaj N | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2021 Journal: Elife / Year: 2021Title: Structural insight into the dual function of LbpB in mediating Neisserial pathogenesis. Authors: Ravi Yadav / Srinivas Govindan / Courtney Daczkowski / Andrew Mesecar / Srinivas Chakravarthy / Nicholas Noinaj /  Abstract: Lactoferrin-binding protein B (LbpB) is a lipoprotein present on the surface of that has been postulated to serve dual functions during pathogenesis in both iron acquisition from lactoferrin (Lf), ...Lactoferrin-binding protein B (LbpB) is a lipoprotein present on the surface of that has been postulated to serve dual functions during pathogenesis in both iron acquisition from lactoferrin (Lf), and in providing protection against the cationic antimicrobial peptide lactoferricin (Lfcn). While previous studies support a dual role for LbpB, exactly how these ligands interact with LbpB has remained unknown. Here, we present the structures of LbpB from and in complex with human holo-Lf, forming a 1:1 complex and confirmed by size-exclusion chromatography small-angle X-ray scattering. LbpB consists of N- and C-lobes with the N-lobe interacting extensively with the C-lobe of Lf. Our structures provide insight into LbpB's preference towards holo-Lf, and our mutagenesis and binding studies show that Lf and Lfcn bind independently. Our studies provide the molecular details for how LbpB serves to capture and preserve Lf in an iron-bound state for delivery to the membrane transporter LbpA for iron piracy, and as an antimicrobial peptide sink to evade host immune defenses. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24233.map.gz emd_24233.map.gz | 59.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24233-v30.xml emd-24233-v30.xml emd-24233.xml emd-24233.xml | 14.8 KB 14.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24233_fsc.xml emd_24233_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_24233.png emd_24233.png | 65.9 KB | ||
| Filedesc metadata |  emd-24233.cif.gz emd-24233.cif.gz | 6.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24233 http://ftp.pdbj.org/pub/emdb/structures/EMD-24233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24233 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24233 | HTTPS FTP |
-Related structure data
| Related structure data |  7n88MC  7jrdC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24233.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24233.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : complex between lactoferrin binding protein and lactoferrin
| Entire | Name: complex between lactoferrin binding protein and lactoferrin |
|---|---|
| Components |
|
-Supramolecule #1: complex between lactoferrin binding protein and lactoferrin
| Supramolecule | Name: complex between lactoferrin binding protein and lactoferrin type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|
-Supramolecule #2: lactoferrin binding protein
| Supramolecule | Name: lactoferrin binding protein / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Neisseria gonorrhoeae (bacteria) Neisseria gonorrhoeae (bacteria) |
-Supramolecule #3: lactoferrin
| Supramolecule | Name: lactoferrin / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Lactoferrin-binding protein B
| Macromolecule | Name: Lactoferrin-binding protein B / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neisseria gonorrhoeae (bacteria) Neisseria gonorrhoeae (bacteria) |
| Molecular weight | Theoretical: 78.432734 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GAMGGNFGVQ PVVESTPTAY PVTFKSKDVP TSPPPAEPSV ETTPVNRPAV GAAMRLLRRN TAFHREDGTA IPDSKQAEEK LSFKEGDVL FLYGSKGNKL QQLKSEIHKR DSDVEIRTSE KENKKYGYEF VDAGYVYTKN GKDEIEQNSG GKRFTHRFGY D GFVYYSGE ...String: GAMGGNFGVQ PVVESTPTAY PVTFKSKDVP TSPPPAEPSV ETTPVNRPAV GAAMRLLRRN TAFHREDGTA IPDSKQAEEK LSFKEGDVL FLYGSKGNKL QQLKSEIHKR DSDVEIRTSE KENKKYGYEF VDAGYVYTKN GKDEIEQNSG GKRFTHRFGY D GFVYYSGE RPSQSLPSAG TVKYFGNWQY MTDAKRHRTG KAVASDDLGY ITFYGNDIGA TSYAAKDADD REKHPAEYTV DF DKKILKG ELIKNQYVQK KNDPKKPLTI YNITADLNGN RFTGSAKVNT EVKTRHADKE YLFFHTDADQ RLEGGFFGDN GEE LAGRFI SNDNGVFGVF AGKQKTNASG TNPAMPFGKH TKILDSLKIS VDEATDENPR PFEVSTMPDF GHPDKLLVEG REIP LVSKE KTIDLADGRK MTVSACCDFL TYVKLGRIKT ERPAVKPKAQ DEEDSGINNG EESEDEEEIA EESEDEVSED DNGED EDEI VEEEADEAEE IEEEAEEEEP EEESPEEGNG VSDGIPPAPE ALKGRDIDLF LKGIRTAEAD IPKTGTAHYT GTWEAR IGE PIQWDNKADK AAKAEFDVDF GNKSISGTLT EQNGVEPAFR IENGVIEGNG FHATARTRDN GINLSGNGST NPQSFKA DN LLVTGGFYGP QAAELGGTIF NKDGKSLGIT EDIENEVENE ADVGEQLEPE VKPQFGVVFG AKKDNKEVEK UniProtKB: Transferrin-binding protein B |
-Macromolecule #2: Lactotransferrin
| Macromolecule | Name: Lactotransferrin / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on peptide bonds (peptidases); Serine endopeptidases |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 76.263266 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GRRRSVQWCA VSQPEATKCF QWQRNMRKVR GPPVSCIKRD SPIQCIQAIA ENRADAVTLD GGFIYEAGLA PYKLRPVAAE VYGTERQPR THYYAVAVVK KGGSFQLNEL QGLKSCHTGL RRTAGWNVPI GTLRPFLNWT GPPEPIEAAV ARFFSASCVP G ADKGQFPN ...String: GRRRSVQWCA VSQPEATKCF QWQRNMRKVR GPPVSCIKRD SPIQCIQAIA ENRADAVTLD GGFIYEAGLA PYKLRPVAAE VYGTERQPR THYYAVAVVK KGGSFQLNEL QGLKSCHTGL RRTAGWNVPI GTLRPFLNWT GPPEPIEAAV ARFFSASCVP G ADKGQFPN LCRLCAGTGE NKCAFSSQEP YFSYSGAFKC LRDGAGDVAF IRESTVFEDL SDEAERDEYE LLCPDNTRKP VD KFKDCHL ARVPSHAVVA RSVNGKEDAI WNLLRQAQEK FGKDKSPKFQ LFGSPSGQKD LLFKDSAIGF SRVPPRIDSG LYL GSGYFT AIQNLRKSEE EVAARRARVV WCAVGEQELR KCNQWSGLSE GSVTCSSAST TEDCIALVLK GEADAMSLDG GYVY TAGKC GLVPVLAENY KSQQSSDPDP NCVDRPVEGY LAVAVVRRSD TSLTWNSVKG KKSCHTAVDR TAGWNIPMGL LFNQT GSCK FDEYFSQSCA PGSDPRSNLC ALCIGDEQGE NKCVPNSNER YYGYTGAFRC LAENAGDVAF VKDVTVLQNT DGNNNE AWA KDLKLADFAL LCLDGKRKPV TEARSCHLAM APNHAVVSRM DKVERLKQVL LHQQAKFGRN GSDCPDKFCL FQSETKN LL FNDNTECLAR LHGKTTYEKY LGPQYVAGIT NLKKCSTSPL LEACEFLRK UniProtKB: Lactotransferrin |
-Macromolecule #3: FE (III) ION
| Macromolecule | Name: FE (III) ION / type: ligand / ID: 3 / Number of copies: 2 / Formula: FE |
|---|---|
| Molecular weight | Theoretical: 55.845 Da |
-Macromolecule #4: BICARBONATE ION
| Macromolecule | Name: BICARBONATE ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: BCT |
|---|---|
| Molecular weight | Theoretical: 61.017 Da |
| Chemical component information |  ChemComp-BCT: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 53.68 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















