+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24115 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of TAX-4_R421W apo open state | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / blindness-associated mutation / achromatopsia / phototransduction / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationdetection of carbon dioxide / detection of chemical stimulus involved in sensory perception / Activation of the phototransduction cascade / Inactivation, recovery and regulation of the phototransduction cascade / VxPx cargo-targeting to cilium / ciliary inversin compartment / multicellular organismal reproductive process / thermosensory behavior / positive regulation of growth rate / G protein-coupled receptor signaling pathway coupled to cGMP nucleotide second messenger ...detection of carbon dioxide / detection of chemical stimulus involved in sensory perception / Activation of the phototransduction cascade / Inactivation, recovery and regulation of the phototransduction cascade / VxPx cargo-targeting to cilium / ciliary inversin compartment / multicellular organismal reproductive process / thermosensory behavior / positive regulation of growth rate / G protein-coupled receptor signaling pathway coupled to cGMP nucleotide second messenger / olfactory behavior / intracellular cyclic nucleotide activated cation channel complex / aerotaxis / intracellularly cGMP-activated cation channel activity / chemosensory behavior / intracellularly cAMP-activated cation channel activity / response to oxygen levels / thermotaxis / cation channel complex / non-motile cilium / regulation of neuron differentiation / regulation of axon extension / negative regulation of receptor guanylyl cyclase signaling pathway / monoatomic cation transmembrane transport / cGMP binding / voltage-gated potassium channel activity / response to hyperoxia / phototransduction / neuron projection morphogenesis / calcium-mediated signaling / chemotaxis / dendrite / positive regulation of gene expression / protein-containing complex binding / positive regulation of transcription by RNA polymerase II / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Zheng X / Li H | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2022 Journal: Commun Biol / Year: 2022Title: Structural and functional characterization of an achromatopsia-associated mutation in a phototransduction channel. Authors: Xiangdong Zheng / Huan Li / Zhengshan Hu / Deyuan Su / Jian Yang /  Abstract: Numerous missense mutations in cyclic nucleotide-gated (CNG) channels cause achromatopsia and retinitis pigmentosa, but the underlying pathogenic mechanisms are often unclear. We investigated the ...Numerous missense mutations in cyclic nucleotide-gated (CNG) channels cause achromatopsia and retinitis pigmentosa, but the underlying pathogenic mechanisms are often unclear. We investigated the structural basis and molecular/cellular effects of R410W, an achromatopsia-associated, presumed loss-of-function mutation in human CNGA3. Cryo-EM structures of the Caenorhabditis elegans TAX-4 CNG channel carrying the analogous mutation, R421W, show that most apo channels are open. R421, located in the gating ring, interacts with the S4 segment in the closed state. R421W disrupts this interaction, destabilizes the closed state, and stabilizes the open state. CNGA3_R410W/CNGB3 and TAX4_R421W channels are spontaneously active without cGMP and induce cell death, suggesting cone degeneration triggered by spontaneous CNG channel activity as a possible cause of achromatopsia. Our study sheds new light on CNG channel allosteric gating, provides an impetus for a reevaluation of reported loss-of-function CNG channel missense disease mutations, and has implications for mutation-specific treatment of retinopathy. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24115.map.gz emd_24115.map.gz | 93.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24115-v30.xml emd-24115-v30.xml emd-24115.xml emd-24115.xml | 12.9 KB 12.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24115.png emd_24115.png | 179.7 KB | ||
| Filedesc metadata |  emd-24115.cif.gz emd-24115.cif.gz | 5.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24115 http://ftp.pdbj.org/pub/emdb/structures/EMD-24115 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24115 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24115 | HTTPS FTP |
-Validation report
| Summary document |  emd_24115_validation.pdf.gz emd_24115_validation.pdf.gz | 502.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24115_full_validation.pdf.gz emd_24115_full_validation.pdf.gz | 501.8 KB | Display | |
| Data in XML |  emd_24115_validation.xml.gz emd_24115_validation.xml.gz | 6.5 KB | Display | |
| Data in CIF |  emd_24115_validation.cif.gz emd_24115_validation.cif.gz | 7.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24115 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24115 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24115 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24115 | HTTPS FTP |
-Related structure data
| Related structure data |  7n17MC  7n15C  7n16C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24115.map.gz / Format: CCP4 / Size: 98.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24115.map.gz / Format: CCP4 / Size: 98.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
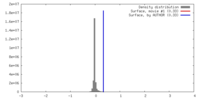
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Cyclic nucleotide-gated channel TAX-4 with R421W mutation
| Entire | Name: Cyclic nucleotide-gated channel TAX-4 with R421W mutation |
|---|---|
| Components |
|
-Supramolecule #1: Cyclic nucleotide-gated channel TAX-4 with R421W mutation
| Supramolecule | Name: Cyclic nucleotide-gated channel TAX-4 with R421W mutation type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Cyclic nucleotide-gated cation channel
| Macromolecule | Name: Cyclic nucleotide-gated cation channel / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 84.334914 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GGGGSMSTAE PAPDPTNPST SGLAPTTNGI GSPPPTASAA TKFSILTKFL RRKNQVHTTT AQQNEFMQKY MPNGNSNAVQ PAATGGQPA SSDGGSAIEV PPPKESYAVR IRKYLANYTQ DPSTDNFYYW TCVVTVAYIY NLLFVIARQV FNDLIGPSSQ S LCRFYNGT ...String: GGGGSMSTAE PAPDPTNPST SGLAPTTNGI GSPPPTASAA TKFSILTKFL RRKNQVHTTT AQQNEFMQKY MPNGNSNAVQ PAATGGQPA SSDGGSAIEV PPPKESYAVR IRKYLANYTQ DPSTDNFYYW TCVVTVAYIY NLLFVIARQV FNDLIGPSSQ S LCRFYNGT LNSTTQVECT YNMLTNMKEM PTYSQYPDLG WSKYWHFRML WVFFDLLMDC VYLIDTFLNY RMGYMDQGLV VR EAEKVTK AYWQSKQYRI DGISLIPLDY ILGWPIPYIN WRGLPILRLN RLIRYKRVRN CLERTETRSS MPNAFRVVVV VWY IVIIIH WNACLYFWIS EWIGLGTDAW VYGHLNKQSL PDDITDTLLR RYVYSFYWST LILTTIGEVP SPVRNIEYAF VTLD LMCGV LIFATIVGNV GSMISNMSAA WTEFQNKMDG IKQYMELRKV SKQLEIRVIK WFDYLWTNKQ SLSDQQVLKV LPDKL QAEI AMQVHFETLR KVRIFQDCEA GLLAELVLKL QLQVFSPGDF ICKKGDIGRE MYIVKRGRLQ VVDDDGKKVF VTLQEG SVF GELSILNIAG SKNGNRRTAN VRSVGYTDLF VLSKTDLWNA LREYPDARKL LLAKGREILK KDNLLDENAP EEQKTVE EI AEHLNNAVKV LQTRMARLIV EHSSTEGKLM KRIEMLEKHL SRYKALARRQ KTMHGVSIDG GDISTDGVDE RVRPPRLR Q TKTIDLPTGT ESESLLK UniProtKB: Cyclic nucleotide-gated channel |
-Macromolecule #2: 1-PALMITOYL-2-LINOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE
| Macromolecule | Name: 1-PALMITOYL-2-LINOLEOYL-SN-GLYCERO-3-PHOSPHOCHOLINE / type: ligand / ID: 2 / Number of copies: 24 / Formula: CPL |
|---|---|
| Molecular weight | Theoretical: 758.06 Da |
| Chemical component information |  ChemComp-CPL: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: C-flat-1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 58.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: OTHER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)