[English] 日本語
 Yorodumi
Yorodumi- EMDB-23913: Cryo-EM reveals partially and fully assembled native glycine rece... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23913 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reveals partially and fully assembled native glycine receptors,heteromeric pentamer | |||||||||
 Map data Map data | unsharpened map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | glycine receptor / ion channel / heteromeric pentamer / MEMBRANE PROTEIN / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationNeurotransmitter receptors and postsynaptic signal transmission / taurine binding / negative regulation of transmission of nerve impulse / positive regulation of acrosome reaction / acrosome reaction / synaptic transmission, glycinergic / glycine-gated chloride channel complex / gamma-aminobutyric acid receptor clustering / neuromuscular process controlling posture / extracellularly glycine-gated ion channel activity ...Neurotransmitter receptors and postsynaptic signal transmission / taurine binding / negative regulation of transmission of nerve impulse / positive regulation of acrosome reaction / acrosome reaction / synaptic transmission, glycinergic / glycine-gated chloride channel complex / gamma-aminobutyric acid receptor clustering / neuromuscular process controlling posture / extracellularly glycine-gated ion channel activity / righting reflex / regulation of respiratory gaseous exchange by nervous system process / extracellularly glycine-gated chloride channel activity / inhibitory synapse / excitatory extracellular ligand-gated monoatomic ion channel activity / glycinergic synapse / adult walking behavior / inhibitory postsynaptic potential / glycine binding / cellular response to zinc ion / startle response / cellular response to ethanol / chloride channel complex / neuropeptide signaling pathway / neuronal action potential / monoatomic ion transport / visual perception / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / chloride transmembrane transport / muscle contraction / cellular response to amino acid stimulus / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / GABA-ergic synapse / transmembrane signaling receptor activity / nervous system development / chemical synaptic transmission / perikaryon / postsynaptic membrane / intracellular membrane-bounded organelle / external side of plasma membrane / dendrite / zinc ion binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Zhu H / Gouaux E | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Architecture and assembly mechanism of native glycine receptors. Authors: Hongtao Zhu / Eric Gouaux /  Abstract: Glycine receptors (GlyRs) are pentameric, 'Cys-loop' receptors that form chloride-permeable channels and mediate fast inhibitory signalling throughout the central nervous system. In the spinal cord ...Glycine receptors (GlyRs) are pentameric, 'Cys-loop' receptors that form chloride-permeable channels and mediate fast inhibitory signalling throughout the central nervous system. In the spinal cord and brainstem, GlyRs regulate locomotion and cause movement disorders when mutated. However, the stoichiometry of native GlyRs and the mechanism by which they are assembled remain unclear, despite extensive investigation. Here we report cryo-electron microscopy structures of native GlyRs from pig spinal cord and brainstem, revealing structural insights into heteromeric receptors and their predominant subunit stoichiometry of 4α:1β. Within the heteromeric pentamer, the β(+)-α(-) interface adopts a structure that is distinct from the α(+)-α(-) and α(+)-β(-) interfaces. Furthermore, the β-subunit contains a unique phenylalanine residue that resides within the pore and disrupts the canonical picrotoxin site. These results explain why inclusion of the β-subunit breaks receptor symmetry and alters ion channel pharmacology. We also find incomplete receptor complexes and, by elucidating their structures, reveal the architectures of partially assembled α-trimers and α-tetramers. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23913.map.gz emd_23913.map.gz | 209.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23913-v30.xml emd-23913-v30.xml emd-23913.xml emd-23913.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23913.png emd_23913.png | 158.3 KB | ||
| Filedesc metadata |  emd-23913.cif.gz emd-23913.cif.gz | 6.7 KB | ||
| Others |  emd_23913_additional_1.map.gz emd_23913_additional_1.map.gz | 12.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23913 http://ftp.pdbj.org/pub/emdb/structures/EMD-23913 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23913 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23913 | HTTPS FTP |
-Related structure data
| Related structure data |  7mlyMC  7mluC  7mlvC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23913.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23913.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | unsharpened map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Localscale sharpened map
| File | emd_23913_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Localscale sharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Native heteromeric glycine receptor bound with 3D1 fab
+Supramolecule #1: Native heteromeric glycine receptor bound with 3D1 fab
+Supramolecule #2: Native heteromeric glycine receptor
+Supramolecule #3: 3D1 fab
+Macromolecule #1: 3D1 Fab Light Chain
+Macromolecule #2: 3D1 Fab Heavy Chain
+Macromolecule #3: Glycine receptor alpha 1
+Macromolecule #4: Glycine receptor beta
+Macromolecule #9: HEPTANE
+Macromolecule #10: nonane
+Macromolecule #11: PENTANE
+Macromolecule #12: PENTADECANE
+Macromolecule #13: DECANE
+Macromolecule #14: N-BUTANE
+Macromolecule #15: GLYCINE
+Macromolecule #16: DODECANE
+Macromolecule #17: N-OCTANE
+Macromolecule #18: HEXANE
+Macromolecule #19: UNDECANE
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.05 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 28.2 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 2.7 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 527075 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: cryoSPARC |
| Final angle assignment | Type: ANGULAR RECONSTITUTION / Software - Name: cryoSPARC |
 Movie
Movie Controller
Controller







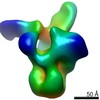





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)