[English] 日本語
 Yorodumi
Yorodumi- EMDB-22814: Mitochondrial Hsp90 (TRAP1) in complex with SdhB folding intermed... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22814 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mitochondrial Hsp90 (TRAP1) in complex with SdhB folding intermediate state in the presence of AMP-PNP | |||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
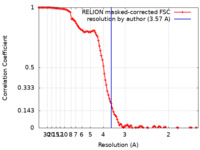
| Method | single particle reconstruction / cryo EM / Resolution: 3.57 Å | |||||||||||||||||||||
 Authors Authors | Liu YX / Agard DA | |||||||||||||||||||||
| Funding support |  United States, 6 items United States, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM reveals the dynamic interplay between mitochondrial Hsp90 and SdhB folding intermediates Authors: Liu YX / Agard DA / Elnatan D / Sun M / Myasnikov AG | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22814.map.gz emd_22814.map.gz | 98.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22814-v30.xml emd-22814-v30.xml emd-22814.xml emd-22814.xml | 16.7 KB 16.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22814_fsc.xml emd_22814_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_22814.png emd_22814.png | 84.3 KB | ||
| Others |  emd_22814_additional_1.map.gz emd_22814_additional_1.map.gz | 117 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22814 http://ftp.pdbj.org/pub/emdb/structures/EMD-22814 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22814 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22814 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22814.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22814.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.814 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: #1
| File | emd_22814_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human Trap1 in complex with SdhB in the presence of AMP-PNP
| Entire | Name: human Trap1 in complex with SdhB in the presence of AMP-PNP |
|---|---|
| Components |
|
-Supramolecule #1: human Trap1 in complex with SdhB in the presence of AMP-PNP
| Supramolecule | Name: human Trap1 in complex with SdhB in the presence of AMP-PNP type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Molecular weight | Experimental: 162 KDa |
-Macromolecule #1: Trap1
| Macromolecule | Name: Trap1 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: GIDPFTSTQT AEDKEEPLHS IISSTESVQG STSKHEFQAE TKKLLDIVAR SLYSEKEVFI RELISNASDA LEKLRHKLVS DGQALPEMEI HLQTNAEKGT ITIQDTGIGM TQEELVSNLG TIARSGSKAF LDALQNQAEA SSKIIGQFGV GFYSAFMVAD RVEVYSRSAA ...String: GIDPFTSTQT AEDKEEPLHS IISSTESVQG STSKHEFQAE TKKLLDIVAR SLYSEKEVFI RELISNASDA LEKLRHKLVS DGQALPEMEI HLQTNAEKGT ITIQDTGIGM TQEELVSNLG TIARSGSKAF LDALQNQAEA SSKIIGQFGV GFYSAFMVAD RVEVYSRSAA PGSLGYQWLS DGSGVFEIAE ASGVRTGTKI IIHLKSDCKE FSSEARVRDV VTKYSNFVSF PLYLNGRRMN TLQAIWMMDP KDVGEWQHEE FYRYVAQAHD KPRYTLHYKT DAPLNIRSIF YVPDMKPSMF DVSRELGSSV ALYSRKVLIQ TKATDILPKW LRFIRGVVDS EDIPLNLSRE LLQESALIRK LRDVLQQRLI KFFIDQSKKD AEKYAKFFED YGLFMREGIV TATEQEVKED IAKLLRYESS ALPSGQLTSL SEYASRMRAG TRNIYYLCAP NRHLAEHSPY YEAMKKKDTE VLFCFEQFDE LTLLHLREFD KKKLISVETD IVVDHYKEEK FEDRSPAAEC LSEKETEELM AWMRNVLGSR VTNVKVTLRL DTHPAMVTVL EMGAARHFLR MQQLAKTQEE RAQLLQPTLE INPRHALIKK LNQLRASEPG LAQLLVDQIY ENAMIAAGLV DDPRAMVGRL NELLVKALER H |
-Macromolecule #2: Trap1
| Macromolecule | Name: Trap1 / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: GIDPFTSTQT AEDKEEPLHS IISSTESVQG STSKHEFQAE TKKLLDIVAR SLYSEKEVFI RELISNASDA LEKLRHKLVS DGQALPEMEI HLQTNAEKGT ITIQDTGIGM TQEELVSNLG TIARSGSKAF LDALQNQAEA SSKIIGQFGV GFYSAFMVAD RVEVYSRSAA ...String: GIDPFTSTQT AEDKEEPLHS IISSTESVQG STSKHEFQAE TKKLLDIVAR SLYSEKEVFI RELISNASDA LEKLRHKLVS DGQALPEMEI HLQTNAEKGT ITIQDTGIGM TQEELVSNLG TIARSGSKAF LDALQNQAEA SSKIIGQFGV GFYSAFMVAD RVEVYSRSAA PGSLGYQWLS DGSGVFEIAE ASGVRTGTKI IIHLKSDCKE FSSEARVRDV VTKYSNFVSF PLYLNGRRMN TLQAIWMMDP KDVGEWQHEE FYRYVAQAHD KPRYTLHYKT DAPLNIRSIF YVPDMKPSMF DVSRELGSSV ALYSRKVLIQ TKATDILPKW LRFIRGVVDS EDIPLNLSRE LLQESALIRK LRDVLQQRLI KFFIDQSKKD AEKYAKFFED YGLFMREGIV TATEQEVKED IAKLLRYESS ALPSGQLTSL SEYASRMRAG TRNIYYLCAP NRHLAEHSPY YEAMKKKDTE VLFCFEQFDE LTLLHLREFD KKKLISVETD IVVDHYKEEK FEDRSPAAEC LSEKETEELM AWMRNVLGSR VTNVKVTLRL DTHPAMVTVL EMGAARHFLR MQQLAKTQEE RAQLLQPTLE INPRHALIKK LNQLRASEPG LAQLLVDQIY ENAMIAAGLV DDPRAMVGRL NELLVKALER H |
-Macromolecule #3: SdhB
| Macromolecule | Name: SdhB / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: GSAQTAAATA PRIKKFAIYR WDPDKAGDKP HMQTYEVDLN KCGPMVLDAL IKIKNEVDST LTFRRSCREG ICGSCAMNIN GGNTLACTRR IDTNLNKVSK IYPLPHMYVI KDLVPDLSNF YAQYKSIEPY LKKK |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 72.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)