[English] 日本語
 Yorodumi
Yorodumi- EMDB-22629: The N-terminus of varicella-zoster virus glycoprotein B has a fun... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22629 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | The N-terminus of varicella-zoster virus glycoprotein B has a functional role in fusion. | |||||||||||||||||||||
 Map data Map data | Cryo-EM map of varicella-zoster virus glycoprotein B purified from infected cells | |||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | Herpesvirus / Varicella-Zoster Virus / Pathogenesis / Fusion / Glycoprotein B / VIRAL PROTEIN | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell endosome / host cell Golgi apparatus / viral envelope / symbiont entry into host cell / virion attachment to host cell / host cell plasma membrane / membrane Similarity search - Function | |||||||||||||||||||||
| Biological species |  Human alphaherpesvirus 3 (Varicella-zoster virus) / Human alphaherpesvirus 3 (Varicella-zoster virus) /  Human herpesvirus 3 (Varicella-zoster virus) Human herpesvirus 3 (Varicella-zoster virus) | |||||||||||||||||||||
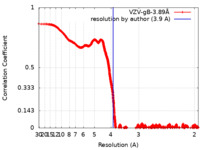
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||||||||||||||
 Authors Authors | Oliver SL | |||||||||||||||||||||
| Funding support |  United States, 6 items United States, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: PLoS Pathog / Year: 2021 Journal: PLoS Pathog / Year: 2021Title: The N-terminus of varicella-zoster virus glycoprotein B has a functional role in fusion. Authors: Stefan L Oliver / Yi Xing / Dong-Hua Chen / Soung Hun Roh / Grigore D Pintilie / David A Bushnell / Marvin H Sommer / Edward Yang / Andrea Carfi / Wah Chiu / Ann M Arvin /   Abstract: Varicella-zoster virus (VZV) is a medically important alphaherpesvirus that induces fusion of the virion envelope and the cell membrane during entry, and between cells to form polykaryocytes within ...Varicella-zoster virus (VZV) is a medically important alphaherpesvirus that induces fusion of the virion envelope and the cell membrane during entry, and between cells to form polykaryocytes within infected tissues during pathogenesis. All members of the Herpesviridae, including VZV, have a conserved core fusion complex composed of glycoproteins, gB, gH and gL. The ectodomain of the primary fusogen, gB, has five domains, DI-V, of which DI contains the fusion loops needed for fusion function. We recently demonstrated that DIV is critical for fusion initiation, which was revealed by a 2.8Å structure of a VZV neutralizing mAb, 93k, bound to gB and mutagenesis of the gB-93k interface. To further assess the mechanism of mAb 93k neutralization, the binding site of a non-neutralizing mAb to gB, SG2, was compared to mAb 93k using single particle cryogenic electron microscopy (cryo-EM). The gB-SG2 interface partially overlapped with that of gB-93k but, unlike mAb 93k, mAb SG2 did not interact with the gB N-terminus, suggesting a potential role for the gB N-terminus in membrane fusion. The gB ectodomain structure in the absence of antibody was defined at near atomic resolution by single particle cryo-EM (3.9Å) of native, full-length gB purified from infected cells and by X-ray crystallography (2.4Å) of the transiently expressed ectodomain. Both structures revealed that the VZV gB N-terminus (aa72-114) was flexible based on the absence of visible structures in the cryo-EM or X-ray crystallography data but the presence of gB N-terminal peptides were confirmed by mass spectrometry. Notably, N-terminal residues 109KSQD112 were predicted to form a small α-helix and alanine substitution of these residues abolished cell-cell fusion in a virus-free assay. Importantly, transferring the 109AAAA112 mutation into the VZV genome significantly impaired viral propagation. These data establish a functional role for the gB N-terminus in membrane fusion broadly relevant to the Herpesviridae. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22629.map.gz emd_22629.map.gz | 228.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22629-v30.xml emd-22629-v30.xml emd-22629.xml emd-22629.xml | 13.9 KB 13.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22629_fsc.xml emd_22629_fsc.xml | 348.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_22629.png emd_22629.png | 121.4 KB | ||
| Filedesc metadata |  emd-22629.cif.gz emd-22629.cif.gz | 6.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22629 http://ftp.pdbj.org/pub/emdb/structures/EMD-22629 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22629 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22629 | HTTPS FTP |
-Validation report
| Summary document |  emd_22629_validation.pdf.gz emd_22629_validation.pdf.gz | 520.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22629_full_validation.pdf.gz emd_22629_full_validation.pdf.gz | 520.1 KB | Display | |
| Data in XML |  emd_22629_validation.xml.gz emd_22629_validation.xml.gz | 70.6 KB | Display | |
| Data in CIF |  emd_22629_validation.cif.gz emd_22629_validation.cif.gz | 145.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22629 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22629 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22629 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22629 | HTTPS FTP |
-Related structure data
| Related structure data |  7k1sMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22629.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22629.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM map of varicella-zoster virus glycoprotein B purified from infected cells | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Varicella-zoster virus glycoprotein B trimer.
| Entire | Name: Varicella-zoster virus glycoprotein B trimer. |
|---|---|
| Components |
|
-Supramolecule #1: Varicella-zoster virus glycoprotein B trimer.
| Supramolecule | Name: Varicella-zoster virus glycoprotein B trimer. / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: The varicella-zoster virus glycoprotein B trimer was purified from infected MeWo cells. |
|---|---|
| Source (natural) | Organism:  Human alphaherpesvirus 3 (Varicella-zoster virus) Human alphaherpesvirus 3 (Varicella-zoster virus) |
-Macromolecule #1: Envelope glycoprotein B
| Macromolecule | Name: Envelope glycoprotein B / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human herpesvirus 3 (Varicella-zoster virus) Human herpesvirus 3 (Varicella-zoster virus) |
| Molecular weight | Theoretical: 105.47325 KDa |
| Sequence | String: MSPCGYYSKW RNRDRPEYRR NLRFRRFFSS IHPNAAAGSG FNGPGVFITS VTGVWLCFLC IFSMFVTAVV SVSPSSFYES LQVEPTQSE DITRSAHLGD GDEIREAIHK SQDAETKPTF YVCPPPTGST IVRLEPPRTC PDYHLGKNFT EGIAVVYKEN I AAYKFKAT ...String: MSPCGYYSKW RNRDRPEYRR NLRFRRFFSS IHPNAAAGSG FNGPGVFITS VTGVWLCFLC IFSMFVTAVV SVSPSSFYES LQVEPTQSE DITRSAHLGD GDEIREAIHK SQDAETKPTF YVCPPPTGST IVRLEPPRTC PDYHLGKNFT EGIAVVYKEN I AAYKFKAT VYYKDVIVST AWAGSSYTQI TNRYADRVPI PVSEITDTID KFGKCSSKAT YVRNNHKVEA FNEDKNPQDM PL IASKYNS VGSKAWHTTN DTYMVAGTPG TYRTGTSVNC IIEEVEARSI FPYDSFGLST GDIIYMSPFF GLRDGAYREH SNY AMDRFH QFEGYRQRDL DTRALLEPAA RNFLVTPHLT VGWNWKPKRT EVCSLVKWRE VEDVVRDEYA HNFRFTMKTL STTF ISETN EFNLNQIHLS QCVKEEARAI INRIYTTRYN SSHVRTGDIQ TYLARGGFVV VFQPLLSNSL ARLYLQELVR ENTNH SPQK HPTRNTRSRR SVPVELRANR TITTTSSVEF AMLQFTYDHI QEHVNEMLAR ISSSWCQLQN RERALWSGLF PINPSA LAS TILDQRVKAR ILGDVISVSN CPELGSDTRI ILQNSMRVSG STTRCYSRPL ISIVSLNGSG TVEGQLGTDN ELIMSRD LL EPCVANHKRY FLFGHHYVYY EDYRYVREIA VHDVGMISTY VDLNLTLLKD REFMPLQVYT RDELRDTGLL DYSEIQRR N QMHSLRFYDI DKVVQYDSGT AIMQGMAQFF QGLGTAGQAV GHVVLGATGA LLSTVHGFTT FLSNPFGALA VGLLVLAGL VAAFFAYRYV LKLKTSPMKA LYPLTTKGLK QLPEGMDPFA EKPNATDTPI EEIGDSQNTE PSVNSGFDPD KFREAQEMIK YMTLVSAAE RQESKARKKN KTSALLTSRL TGLALRNRRG YSRVRTENVT GV UniProtKB: Envelope glycoprotein B |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 3 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 7.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)