[English] 日本語
 Yorodumi
Yorodumi- EMDB-22046: The mouse cGAS catalytic domain binding to human assembled nucleosome -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22046 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
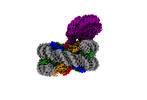
| Title | The mouse cGAS catalytic domain binding to human assembled nucleosome | |||||||||
 Map data Map data | mcGAS catalytic domain with human assembled nucleosome | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Immunity / DNA BINDING PROTEIN-DNA-TRANSFERASE complex | |||||||||
| Function / homology |  Function and homology information Function and homology information2',3'-cyclic GMP-AMP synthase activity / cyclic GMP-AMP synthase / regulation of type I interferon production / paracrine signaling / poly-ADP-D-ribose modification-dependent protein binding / regulation of immunoglobulin production / cGAS/STING signaling pathway / regulation of T cell activation / : / : ...2',3'-cyclic GMP-AMP synthase activity / cyclic GMP-AMP synthase / regulation of type I interferon production / paracrine signaling / poly-ADP-D-ribose modification-dependent protein binding / regulation of immunoglobulin production / cGAS/STING signaling pathway / regulation of T cell activation / : / : / negative regulation of DNA repair / negative regulation of cGAS/STING signaling pathway / cellular response to exogenous dsRNA / regulation of immune response / negative regulation of double-strand break repair via homologous recombination / nucleosome binding / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / positive regulation of type I interferon production / positive regulation of defense response to virus by host / CENP-A containing nucleosome / phosphatidylinositol-4,5-bisphosphate binding / Packaging Of Telomere Ends / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / activation of innate immune response / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / determination of adult lifespan / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / innate immune response in mucosa / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / Negative Regulation of CDH1 Gene Transcription / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / molecular condensate scaffold activity / Nonhomologous End-Joining (NHEJ) / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / HDMs demethylate histones / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Metalloprotease DUBs / Transcriptional regulation of granulopoiesis / RMTs methylate histone arginines / HCMV Early Events / structural constituent of chromatin / positive regulation of cellular senescence / UCH proteinases / heterochromatin formation / nucleosome / antimicrobial humoral immune response mediated by antimicrobial peptide / nucleosome assembly / E3 ubiquitin ligases ubiquitinate target proteins / antibacterial humoral response / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / site of double-strand break / HATs acetylate histones / Factors involved in megakaryocyte development and platelet production / RUNX1 regulates transcription of genes involved in differentiation of HSCs / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / chromatin organization / Processing of DNA double-strand break ends / Senescence-Associated Secretory Phenotype (SASP) / double-stranded DNA binding / gene expression / Oxidative Stress Induced Senescence / Estrogen-dependent gene expression / defense response to virus / chromosome, telomeric region / Ub-specific processing proteases / nuclear body / defense response to Gram-positive bacterium Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.98 Å | |||||||||
 Authors Authors | Pengbiao X / Pingwei L | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: The molecular basis of tight nuclear tethering and inactivation of cGAS. Authors: Baoyu Zhao / Pengbiao Xu / Chesley M Rowlett / Tao Jing / Omkar Shinde / Yuanjiu Lei / A Phillip West / Wenshe Ray Liu / Pingwei Li /  Abstract: Nucleic acids derived from pathogens induce potent innate immune responses. Cyclic GMP-AMP synthase (cGAS) is a double-stranded DNA sensor that catalyses the synthesis of the cyclic dinucleotide ...Nucleic acids derived from pathogens induce potent innate immune responses. Cyclic GMP-AMP synthase (cGAS) is a double-stranded DNA sensor that catalyses the synthesis of the cyclic dinucleotide cyclic GMP-AMP, which mediates the induction of type I interferons through the STING-TBK1-IRF3 signalling axis. cGAS was previously thought to not react with self DNA owing to its cytosolic localization; however, recent studies have shown that cGAS is localized mostly in the nucleus and has low activity as a result of tight nuclear tethering. Here we show that cGAS binds to nucleosomes with nanomolar affinity and that nucleosome binding potently inhibits its catalytic activity. To elucidate the molecular basis of cGAS inactivation by nuclear tethering, we determined the structure of mouse cGAS bound to human nucleosome by cryo-electron microscopy. The structure shows that cGAS binds to a negatively charged acidic patch formed by histones H2A and H2B via its second DNA-binding site. High-affinity nucleosome binding blocks double-stranded DNA binding and maintains cGAS in an inactive conformation. Mutations of cGAS that disrupt nucleosome binding alter cGAS-mediated signalling in cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22046.map.gz emd_22046.map.gz | 4.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22046-v30.xml emd-22046-v30.xml emd-22046.xml emd-22046.xml | 22 KB 22 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22046_fsc.xml emd_22046_fsc.xml | 7.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_22046.png emd_22046.png | 84.9 KB | ||
| Filedesc metadata |  emd-22046.cif.gz emd-22046.cif.gz | 7.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22046 http://ftp.pdbj.org/pub/emdb/structures/EMD-22046 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22046 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22046 | HTTPS FTP |
-Related structure data
| Related structure data |  6x59MC  6x5aC  6xjdC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10513 (Title: mouse cGAS with reconstituted nucleosome / Data size: 2.8 TB EMPIAR-10513 (Title: mouse cGAS with reconstituted nucleosome / Data size: 2.8 TBData #1: Unaligned multiframe micrographs of mouse cGAS / human reconstituted nucleosomes [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22046.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22046.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | mcGAS catalytic domain with human assembled nucleosome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : cGAS-nucleosome complex
| Entire | Name: cGAS-nucleosome complex |
|---|---|
| Components |
|
-Supramolecule #1: cGAS-nucleosome complex
| Supramolecule | Name: cGAS-nucleosome complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#7 Details: The mouse cGAS catalytic domain binding to assembled human nucleosome |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Histone H3.2
| Macromolecule | Name: Histone H3.2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.257838 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ARTKQTARKS TGGKAPRKQL ATKAARKSAP ATGGVKKPHR YRPGTVALRE IRRYQKSTEL LIRKLPFQRL VREIAQDFKT DLRFQSSAV MALQEASEAY LVGLFEDTNL AAIHAKRVTI MPKDIQLARR IRGERA UniProtKB: Histone H3.2 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.263231 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SGRGKGGKGL GKGGAKRHRK VLRDNIQGIT KPAIRRLARR GGVKRISGLI YEETRGVLKV FLENVIRDAV TYTEHAKRKT VTAMDVVYA LKRQGRTLYG FGG UniProtKB: Histone H4 |
-Macromolecule #3: Histone H2A type 1
| Macromolecule | Name: Histone H2A type 1 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.990342 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SGRGKQGGKA RAKAKTRSSR AGLQFPVGRV HRLLRKGNYA ERVGAGAPVY LAAVLEYLTA EILELAGNAA RDNKKTRIIP RHLQLAIRN DEELNKLLGK VTIAQGGVLP NIQAVLLPKK TESHHKAKGK UniProtKB: Histone H2A type 1 |
-Macromolecule #4: Histone H2B type 1-C/E/F/G/I
| Macromolecule | Name: Histone H2B type 1-C/E/F/G/I / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.79598 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SEPAKSAPAP KKGSKKAVTK AQKKDGKKRK RSRKESYSVY VYKVLKQVHP DTGISSKAMG IMNSFVNDIF ERIAGEASRL AHYNKRSTI TSREIQTAVR LLLPGELAKH AVSEGTKAVT KYTSSK UniProtKB: Histone H2B type 1-C/E/F/G/I |
-Macromolecule #7: Cyclic GMP-AMP synthase
| Macromolecule | Name: Cyclic GMP-AMP synthase / type: protein_or_peptide / ID: 7 / Number of copies: 1 / Enantiomer: LEVO / EC number: cyclic GMP-AMP synthase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 43.647352 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSEFELGSRK EPDKLKKVLD KLRLKRKDIS EAAETVNKVV ERLLRRMQKR ESEFKGVEQL NTGSYYEHVK ISAPNEFDVM FKLEVPRIE LQEYYETGAF YLVKFKRIPR GNPLSHFLEG EVLSATKMLS KFRKIIKEEV KEIKDIDVSV EKEKPGSPAV T LLIRNPEE ...String: GSEFELGSRK EPDKLKKVLD KLRLKRKDIS EAAETVNKVV ERLLRRMQKR ESEFKGVEQL NTGSYYEHVK ISAPNEFDVM FKLEVPRIE LQEYYETGAF YLVKFKRIPR GNPLSHFLEG EVLSATKMLS KFRKIIKEEV KEIKDIDVSV EKEKPGSPAV T LLIRNPEE ISVDIILALE SKGSWPISTK EGLPIQGWLG TKVRTNLRRE PFYLVPKNAK DGNSFQGETW RLSFSHTEKY IL NNHGIEK TCCESSGAKC CRKECLKLMK YLLEQLKKEF QELDAFCSYH VKTAIFHMWT QDPQDSQWDP RNLSSCFDKL LAF FLECLR TEKLDHYFIP KFNLFSQELI DRKSKEFLSK KIEYERNNGF PIFDKL UniProtKB: Cyclic GMP-AMP synthase |
-Macromolecule #5: DNA
| Macromolecule | Name: DNA / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.145754 KDa |
| Sequence | String: (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA) (DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA)(DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA) (DG) (DA)(DC)(DA)(DG)(DC)(DT) ...String: (DC)(DT)(DG)(DG)(DA)(DG)(DA)(DA)(DT)(DC) (DC)(DC)(DG)(DG)(DT)(DG)(DC)(DC)(DG)(DA) (DG)(DG)(DC)(DC)(DG)(DC)(DT)(DC)(DA) (DA)(DT)(DT)(DG)(DG)(DT)(DC)(DG)(DT)(DA) (DG) (DA)(DC)(DA)(DG)(DC)(DT)(DC)(DT) (DA)(DG)(DC)(DA)(DC)(DC)(DG)(DC)(DT)(DT) (DA)(DA) (DA)(DC)(DG)(DC)(DA)(DC)(DG) (DT)(DA)(DC)(DG)(DC)(DG)(DC)(DT)(DG)(DT) (DC)(DC)(DC) (DC)(DC)(DG)(DC)(DG)(DT) (DT)(DT)(DT)(DA)(DA)(DC)(DC)(DG)(DC)(DC) (DA)(DA)(DG)(DG) (DG)(DG)(DA)(DT)(DT) (DA)(DC)(DT)(DC)(DC)(DC)(DT)(DA)(DG)(DT) (DC)(DT)(DC)(DC)(DA) (DG)(DG)(DC)(DA) (DC)(DG)(DT)(DG)(DT)(DC)(DA)(DG)(DA)(DT) (DA)(DT)(DA)(DT)(DA)(DC) (DA)(DT)(DC) (DC)(DT)(DG)(DT) |
-Macromolecule #6: DNA
| Macromolecule | Name: DNA / type: dna / ID: 6 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 45.604047 KDa |
| Sequence | String: (DA)(DC)(DA)(DG)(DG)(DA)(DT)(DG)(DT)(DA) (DT)(DA)(DT)(DA)(DT)(DC)(DT)(DG)(DA)(DC) (DA)(DC)(DG)(DT)(DG)(DC)(DC)(DT)(DG) (DG)(DA)(DG)(DA)(DC)(DT)(DA)(DG)(DG)(DG) (DA) (DG)(DT)(DA)(DA)(DT)(DC) ...String: (DA)(DC)(DA)(DG)(DG)(DA)(DT)(DG)(DT)(DA) (DT)(DA)(DT)(DA)(DT)(DC)(DT)(DG)(DA)(DC) (DA)(DC)(DG)(DT)(DG)(DC)(DC)(DT)(DG) (DG)(DA)(DG)(DA)(DC)(DT)(DA)(DG)(DG)(DG) (DA) (DG)(DT)(DA)(DA)(DT)(DC)(DC)(DC) (DC)(DT)(DT)(DG)(DG)(DC)(DG)(DG)(DT)(DT) (DA)(DA) (DA)(DA)(DC)(DG)(DC)(DG)(DG) (DG)(DG)(DG)(DA)(DC)(DA)(DG)(DC)(DG)(DC) (DG)(DT)(DA) (DC)(DG)(DT)(DG)(DC)(DG) (DT)(DT)(DT)(DA)(DA)(DG)(DC)(DG)(DG)(DT) (DG)(DC)(DT)(DA) (DG)(DA)(DG)(DC)(DT) (DG)(DT)(DC)(DT)(DA)(DC)(DG)(DA)(DC)(DC) (DA)(DA)(DT)(DT)(DG) (DA)(DG)(DC)(DG) (DG)(DC)(DC)(DT)(DC)(DG)(DG)(DC)(DA)(DC) (DC)(DG)(DG)(DG)(DA)(DT) (DT)(DC)(DT) (DC)(DC)(DA)(DG) |
-Macromolecule #8: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 8 / Number of copies: 1 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 298 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















