[English] 日本語
 Yorodumi
Yorodumi- EMDB-22004: +3 extended HIV-1 reverse transcriptase initiation complex (inter... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22004 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | +3 extended HIV-1 reverse transcriptase initiation complex (intermediate state, unmasked map) | |||||||||
 Map data Map data | +3 extended HIV-1 reverse transcriptase initiation complex | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.3 Å | |||||||||
 Authors Authors | Larsen KP / Jackson LN / Zhang J / Puglisi EV | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: J Mol Biol / Year: 2020 Journal: J Mol Biol / Year: 2020Title: Distinct Conformational States Underlie Pausing during Initiation of HIV-1 Reverse Transcription. Authors: Kevin P Larsen / Junhong Choi / Lynnette N Jackson / Kalli Kappel / Jingji Zhang / Betty Ha / Dong-Hua Chen / Elisabetta Viani Puglisi /  Abstract: A hallmark of the initiation step of HIV-1 reverse transcription, in which viral RNA genome is converted into double-stranded DNA, is that it is slow and non-processive. Biochemical studies have ...A hallmark of the initiation step of HIV-1 reverse transcription, in which viral RNA genome is converted into double-stranded DNA, is that it is slow and non-processive. Biochemical studies have identified specific sites along the viral RNA genomic template in which reverse transcriptase (RT) stalls. These stalling points, which occur after the addition of three and five template dNTPs, may serve as checkpoints to regulate the precise timing of HIV-1 reverse transcription following viral entry. Structural studies of reverse transcriptase initiation complexes (RTICs) have revealed unique conformations that may explain the slow rate of incorporation; however, questions remain about the temporal evolution of the complex and features that contribute to strong pausing during initiation. Here we present cryo-electron microscopy and single-molecule characterization of an RTIC after three rounds of dNTP incorporation (+3), the first major pausing point during reverse transcription initiation. Cryo-electron microscopy structures of a +3 extended RTIC reveal conformational heterogeneity within the RTIC core. Three distinct conformations were identified, two of which adopt unique, likely off-pathway, intermediates in the canonical polymerization cycle. Single-molecule Förster resonance energy transfer experiments confirm that the +3 RTIC is more structurally dynamic than earlier-stage RTICs. These alternative conformations were selectively disrupted through structure-guided point mutations to shift single-molecule Förster resonance energy transfer populations back toward the on-pathway conformation. Our results support the hypothesis that conformational heterogeneity within the HIV-1 RTIC during pausing serves as an additional means of regulating HIV-1 replication. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22004.map.gz emd_22004.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22004-v30.xml emd-22004-v30.xml emd-22004.xml emd-22004.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22004.png emd_22004.png | 28 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22004 http://ftp.pdbj.org/pub/emdb/structures/EMD-22004 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22004 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22004 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22004.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22004.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | +3 extended HIV-1 reverse transcriptase initiation complex | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
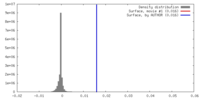
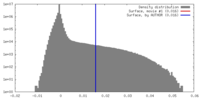
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : +3 extended HIV-1 reverse transcriptase initiation complex (inter...
| Entire | Name: +3 extended HIV-1 reverse transcriptase initiation complex (intermediate state) |
|---|---|
| Components |
|
-Supramolecule #1: +3 extended HIV-1 reverse transcriptase initiation complex (inter...
| Supramolecule | Name: +3 extended HIV-1 reverse transcriptase initiation complex (intermediate state) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 25 KDa |
-Supramolecule #2: HIV-1 reverse transcriptase
| Supramolecule | Name: HIV-1 reverse transcriptase / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 Details: The C-terminus of p66 contains an unstructured linker and a six-histidine tag that was cleaved prior to full complex formation. |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Recombinant expression | Organism:  |
-Supramolecule #3: HIV-1 RNA genome fragment
| Supramolecule | Name: HIV-1 RNA genome fragment / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 Details: A fragment of the HIV-1 RNA genome that is 101 nucleotides in length. |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Recombinant expression | Organism:  |
-Supramolecule #4: tRNA lysine 3
| Supramolecule | Name: tRNA lysine 3 / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #4 Details: Chemically synthesized and extended tRNA lysine 3. A modified dG containing an N2-cystamine was placed at position 71 and the sequence was extended on the 3' end by a dC, dT, and ddG to ...Details: Chemically synthesized and extended tRNA lysine 3. A modified dG containing an N2-cystamine was placed at position 71 and the sequence was extended on the 3' end by a dC, dT, and ddG to bring its total length to 79 nucleotides. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.0 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: Full complex was prepared 5-8 hours before freezing. Beta-OG was added just prior to freezing. | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 292 K / Instrument: LEICA EM GP / Details: 3 microliters applied, 2s pre-blot, 2.5s blot.. | |||||||||||||||
| Details | Sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 77.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)