+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21205 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Teneurin-2 and human Latrophilin-3 binary complex | ||||||||||||
 Map data Map data | Teneurin-Latrophilin complex containing domain 1-5 of human Teneurine-2 and Lectin domain of human Latrophilin-3 | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Adhesion GPCR / synapse / Teneurin / Lphn / MEMBRANE PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationretrograde trans-synaptic signaling by trans-synaptic protein complex / Rho-activating G protein-coupled receptor signaling pathway / cell adhesion mediator activity / excitatory synapse assembly / : / positive regulation of filopodium assembly / neuron development / synapse assembly / cell adhesion molecule binding / filopodium ...retrograde trans-synaptic signaling by trans-synaptic protein complex / Rho-activating G protein-coupled receptor signaling pathway / cell adhesion mediator activity / excitatory synapse assembly / : / positive regulation of filopodium assembly / neuron development / synapse assembly / cell adhesion molecule binding / filopodium / molecular condensate scaffold activity / cell-cell adhesion / PML body / G protein-coupled receptor activity / adenylate cyclase-activating G protein-coupled receptor signaling pathway / neuron migration / cell-cell junction / cell junction / growth cone / carbohydrate binding / dendritic spine / postsynaptic membrane / cell surface receptor signaling pathway / neuron projection / G protein-coupled receptor signaling pathway / protein heterodimerization activity / signaling receptor binding / axon / calcium ion binding / synapse / dendrite / glutamatergic synapse / negative regulation of transcription by RNA polymerase II / endoplasmic reticulum / Golgi apparatus / signal transduction / protein homodimerization activity / nucleus / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
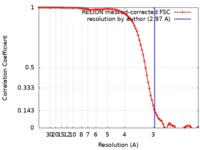
| Method | single particle reconstruction / cryo EM / Resolution: 2.97 Å | ||||||||||||
 Authors Authors | Xie Y / Li J | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: Alternative splicing controls teneurin-latrophilin interaction and synapse specificity by a shape-shifting mechanism. Authors: Jingxian Li / Yuan Xie / Shaleeka Cornelius / Xian Jiang / Richard Sando / Szymon P Kordon / Man Pan / Katherine Leon / Thomas C Südhof / Minglei Zhao / Demet Araç /  Abstract: The trans-synaptic interaction of the cell-adhesion molecules teneurins (TENs) with latrophilins (LPHNs/ADGRLs) promotes excitatory synapse formation when LPHNs simultaneously interact with FLRTs. ...The trans-synaptic interaction of the cell-adhesion molecules teneurins (TENs) with latrophilins (LPHNs/ADGRLs) promotes excitatory synapse formation when LPHNs simultaneously interact with FLRTs. Insertion of a short alternatively-spliced region within TENs abolishes the TEN-LPHN interaction and switches TEN function to specify inhibitory synapses. How alternative-splicing regulates TEN-LPHN interaction remains unclear. Here, we report the 2.9 Å resolution cryo-EM structure of the TEN2-LPHN3 complex, and describe the trimeric TEN2-LPHN3-FLRT3 complex. The structure reveals that the N-terminal lectin domain of LPHN3 binds to the TEN2 barrel at a site far away from the alternatively spliced region. Alternative-splicing regulates the TEN2-LPHN3 interaction by hindering access to the LPHN-binding surface rather than altering it. Strikingly, mutagenesis of the LPHN-binding surface of TEN2 abolishes the LPHN3 interaction and impairs excitatory but not inhibitory synapse formation. These results suggest that a multi-level coincident binding mechanism mediated by a cryptic adhesion complex between TENs and LPHNs regulates synapse specificity. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21205.map.gz emd_21205.map.gz | 49.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21205-v30.xml emd-21205-v30.xml emd-21205.xml emd-21205.xml | 27.2 KB 27.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21205_fsc.xml emd_21205_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_21205.png emd_21205.png | 78.9 KB | ||
| Filedesc metadata |  emd-21205.cif.gz emd-21205.cif.gz | 8.1 KB | ||
| Others |  emd_21205_additional_1.map.gz emd_21205_additional_1.map.gz emd_21205_additional_2.map.gz emd_21205_additional_2.map.gz emd_21205_additional_3.map.gz emd_21205_additional_3.map.gz emd_21205_half_map_1.map.gz emd_21205_half_map_1.map.gz emd_21205_half_map_2.map.gz emd_21205_half_map_2.map.gz | 49.6 MB 60 MB 60 MB 49.7 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21205 http://ftp.pdbj.org/pub/emdb/structures/EMD-21205 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21205 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21205 | HTTPS FTP |
-Validation report
| Summary document |  emd_21205_validation.pdf.gz emd_21205_validation.pdf.gz | 794.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_21205_full_validation.pdf.gz emd_21205_full_validation.pdf.gz | 793.8 KB | Display | |
| Data in XML |  emd_21205_validation.xml.gz emd_21205_validation.xml.gz | 16.1 KB | Display | |
| Data in CIF |  emd_21205_validation.cif.gz emd_21205_validation.cif.gz | 21.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21205 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21205 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21205 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-21205 | HTTPS FTP |
-Related structure data
| Related structure data |  6vhhMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21205.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21205.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Teneurin-Latrophilin complex containing domain 1-5 of human Teneurine-2 and Lectin domain of human Latrophilin-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Refined map focusing on Domain 3
| File | emd_21205_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Refined map focusing on Domain 3 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Postprocessed map (sharpened and filtered)
| File | emd_21205_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Postprocessed map (sharpened and filtered) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Sharpened map for main entry
| File | emd_21205_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map for main entry | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2
| File | emd_21205_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1
| File | emd_21205_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Binary complex of human Teneurin-2 with human Latrophilin-3 extra...
| Entire | Name: Binary complex of human Teneurin-2 with human Latrophilin-3 extracellular domain |
|---|---|
| Components |
|
-Supramolecule #1: Binary complex of human Teneurin-2 with human Latrophilin-3 extra...
| Supramolecule | Name: Binary complex of human Teneurin-2 with human Latrophilin-3 extracellular domain type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 Details: The binary complex includes human Teneurin2 (TENM2) (Teneurin without alternative splice sequence NKEFKHS) and human Latrophilin3 (LPHN3/ADGRL3) with an alternative splice sequence KVEQK ...Details: The binary complex includes human Teneurin2 (TENM2) (Teneurin without alternative splice sequence NKEFKHS) and human Latrophilin3 (LPHN3/ADGRL3) with an alternative splice sequence KVEQK between lectin domain and olfactomedin domain. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 314 KDa |
-Macromolecule #1: Teneurin-2
| Macromolecule | Name: Teneurin-2 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 216.254578 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: TSCADNKDNE GDGLVDCLDP DCCLQSACQN SLLCRGSRDP LDIIQQGQTD WPAVKSFYDR IKLLAGKDST HIIPGENPFN SSLVSLIRG QVVTTDGTPL VGVNVSFVKY PKYGYTITRQ DGTFDLIANG GASLTLHFER APFMSQERTV WLPWNSFYAM D TLVMKTEE ...String: TSCADNKDNE GDGLVDCLDP DCCLQSACQN SLLCRGSRDP LDIIQQGQTD WPAVKSFYDR IKLLAGKDST HIIPGENPFN SSLVSLIRG QVVTTDGTPL VGVNVSFVKY PKYGYTITRQ DGTFDLIANG GASLTLHFER APFMSQERTV WLPWNSFYAM D TLVMKTEE NSIPSCDLSG FVRPDPIIIS SPLSTFFSAA PGQNPIVPET QVLHEEIELP GSNVKLRYLS SRTAGYKSLL KI TMTQSTV PLNLIRVHLM VAVEGHLFQK SFQASPNLAY TFIWDKTDAY GQRVYGLSDA VVSVGFEYET CPSLILWEKR TAL LQGFEL DPSNLGGWSL DKHHILNVKS GILHKGTGEN QFLTQQPAII TSIMGNGRRR SISCPSCNGL AEGNKLLAPV ALAV GIDGS LYVGDFNYIR RIFPSRNVTS ILELRNNPAH KYYLAVDPVS GSLYVSDTNS RRIYRVKSLS GTKDLAGNSE VVAGT GEQC LPFDEARCGD GGKAIDATLM SPRGIAVDKN GLMYFVDATM IRKVDQNGII STLLGSNDLT AVRPLSCDSS MDVAQV RLE WPTDLAVNPM DNSLYVLENN VILRITENHQ VSIIAGRPMH CQVPGIDYSL SKLAIHSALE SASAIAISHT GVLYITE TD EKKINRLRQV TTNGEICLLA GAASDCDCKN DVNCNCYSGD DAYATDAILN SPSSLAVAPD GTIYIADLGN IRIRAVSK N KPVLNAFNQY EAASPGEQEL YVFNADGIHQ YTVSLVTGEY LYNFTYSTDN DVTELIDNNG NSLKIRRDSS GMPRHLLMP DNQIITLTVG TNGGLKVVST QNLELGLMTY DGNTGLLATK SDETGWTTFY DYDHEGRLTN VTRPTGVVTS LHREMEKSIT IDIENSNRD DDVTVITNLS SVEASYTVVQ DQVRNSYQLC NNGTLRVMYA NGMGISFHSE PHVLAGTITP TIGRCNISLP M ENGLNSIE WRLRKEQIKG KVTIFGRKLR VHGRNLLSID YDRSIRTEKI YDDHRKFTLR IIYDQVGRPF LWLPSSGLAA VN VSYFFNG RLAGLQRGAM SERTDIDKQG RIVSRMFADG KVWSYSYLDK SMVLLLQSQR QYIFEYDSSD RLLAVTMPSV ARH SMSTHT SIGYIRNIYN PPESNASVIF DYSDDGRILK TSFLGTGRQV FYKYGKLSKL SEIVYDSTAV TFGYDETTGV LKMV NLQSG GFSCTIRYRK IGPLVDKQIY RFSEEGMVNA RFDYTYHDNS FRIASIKPVI SETPLPVDLY RYDEISGKVE HFGKF GVIY YDINQIITTA VMTLSKHFDT HGRIKEVQYE MFRSLMYWMT VQYDSMGRVI KRELKLGPYA NTTKYTYDYD GDGQLQ SVA VNDRPTWRYS YDLNGNLHLL NPGNSVRLMP LRYDLRDRIT RLGDVQYKID DDGYLCQRGS DIFEYNSKGL LTRAYNK AS GWSVQYRYDG VGRRASYKTN LGHHLQYFYS DLHNPTRITH VYNHSNSEIT SLYYDLQGHL FAMESSSGEE YYVASDNT G TPLAVFSING LMIKQLQYTA YGEIYYDSNP DFQMVIGFHG GLYDPLTKLV HFTQRDYDVL AGRWTSPDYT MWKNVGKEP APFNLYMFKS NNPLSSELGL KNYVTDVKSW LVMFGFQLSN IIPGFPRAKM YFVPPPYELS ESQASENGQL ITGVQQKTER HNQAFMALE GQVITKKLHA SIREKAGHWF ATTTPIIGKG IMFAIKEGRV TTGVSSIASE DSRKVASVLN NAYYLDKMHY S IEGKDTHY FVKIGSADGD LVTLGTTIGR KVLESGVNVT VSQPTLLVNG RTRRFTNIEF QYSTLLLSIR YGLTPDTLDE EK ARVLDQA RQRALGTAWA KEQQKARDGR EGSRLWTEGE KQQLLSTGRV QGYEGYYVLP VEQYPELADS SSNIQFLRQN EMG KRHHHH HH UniProtKB: Teneurin-2 |
-Macromolecule #2: Adhesion G protein-coupled receptor L3
| Macromolecule | Name: Adhesion G protein-coupled receptor L3 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 95.209586 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: SRAPIPMAVV RRELSCESYP IELRCPGTDV IMIESANYGR TDDKICDSDP AQMENIRCYL PDAYKIMSQR CNNRTQCAVV AGPDVFPDP CPGTYKYLEV QYECVPYKVE QKVFLCPGLL KGVYQSEHLF ESDHQSGAWC KDPLQASDKI YYMPWTPYRT D TLTEYSSK ...String: SRAPIPMAVV RRELSCESYP IELRCPGTDV IMIESANYGR TDDKICDSDP AQMENIRCYL PDAYKIMSQR CNNRTQCAVV AGPDVFPDP CPGTYKYLEV QYECVPYKVE QKVFLCPGLL KGVYQSEHLF ESDHQSGAWC KDPLQASDKI YYMPWTPYRT D TLTEYSSK DDFIAGRPTT TYKLPHRVDG TGFVVYDGAL FFNKERTRNI VKFDLRTRIK SGEAIIANAN YHDTSPYRWG GK SDIDLAV DENGLWVIYA TEQNNGKIVI SQLNPYTLRI EGTWDTAYDK RSASNAFMIC GILYVVKSVY EDDDNEATGN KID YIYNTD QSKDSLVDVP FPNSYQYIAA VDYNPRDNLL YVWNNYHVVK YSLDFGPLDS RSGQAHHGQV SYISPPIHLD SELE RPSVK DISTTGPLGM GSTTTSTTLR TTTLSPGRST TPSVSGRRNR STSTPSPAVE VLDDMTTHLP SASSQIPALE ESCEA VEAR EIMWFKTRQG QIAKQPCPAG TIGVSTYLCL APDGIWDPQG PDLSNCSSPW VNHITQKLKS GETAANIARE LAEQTR NHL NAGDITYSVR AMDQLVGLLD VQLRNLTPGG KDSAARSLNK AMVETVNNLL QPQALNAWRD LTTSDQLRAA TMLLHTV EE SAFVLADNLL KTDIVRENTD NIKLEVARLS TEGNLEDLKF PENMGHGSTI QLSANTLKQN GRNGEIRVAF VLYNNLGP Y LSTENASMKL GTEALSTNHS VIVNSPVITA AINKEFSNKV YLADPVVFTV KHIKQSEENF NPNCSFWSYS KRTMTGYWS TQGCRLLTTN KTHTTCSCNH LTNFAVLMAH VEVKHSDAVH DLLLDVHHHH HH UniProtKB: Adhesion G protein-coupled receptor L3 |
-Macromolecule #6: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 6 / Number of copies: 3 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2.2 mg/mL |
|---|---|
| Buffer | pH: 8.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number real images: 5402 / Average exposure time: 4.2 sec. / Average electron dose: 60.1 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)