+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21132 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
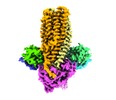
| Title | Cryo-EM structure of PCAT1 bound to its CtA peptide substrate | |||||||||
 Map data Map data | full map from cryosparc | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ATP-Binding Cassette / PROTEIN TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationABC-type bacteriocin transporter activity / ABC-type oligopeptide transporter activity / cysteine-type peptidase activity / ATP hydrolysis activity / proteolysis / ATP binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Hungateiclostridium thermocellum (bacteria) / Hungateiclostridium thermocellum (bacteria) /  Hungateiclostridium thermocellum (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) (bacteria) Hungateiclostridium thermocellum (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) (bacteria) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.35 Å | |||||||||
 Authors Authors | Kieuvongngam V / Oldham ML | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2020 Journal: Elife / Year: 2020Title: Structural basis of substrate recognition by a polypeptide processing and secretion transporter. Authors: Virapat Kieuvongngam / Paul Dominic B Olinares / Anthony Palillo / Michael L Oldham / Brian T Chait / Jue Chen /  Abstract: The peptidase-containing ATP-binding cassette transporters (PCATs) are unique members of the ABC transporter family that proteolytically process and export peptides and proteins. Each PCAT contains ...The peptidase-containing ATP-binding cassette transporters (PCATs) are unique members of the ABC transporter family that proteolytically process and export peptides and proteins. Each PCAT contains two peptidase domains that cleave off the secretion signal, two transmembrane domains forming a translocation pathway, and two nucleotide-binding domains that hydrolyze ATP. Previously the crystal structures of a PCAT from (PCAT1) were determined in the absence and presence of ATP, revealing how ATP binding regulates the protease activity and access to the translocation pathway. However, how the substrate CtA, a 90-residue polypeptide, is recognized by PCAT1 remained elusive. To address this question, we determined the structure of the PCAT1-CtA complex by electron cryo-microscopy (cryo-EM) to 3.4 Å resolution. The structure shows that two CtAs are bound via their N-terminal leader peptides, but only one is positioned for cleavage and translocation. Based on these results, we propose a model of how substrate cleavage, ATP hydrolysis, and substrate translocation are coordinated in a transport cycle. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21132.map.gz emd_21132.map.gz | 52 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21132-v30.xml emd-21132-v30.xml emd-21132.xml emd-21132.xml | 22.5 KB 22.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21132.png emd_21132.png | 113.1 KB | ||
| Filedesc metadata |  emd-21132.cif.gz emd-21132.cif.gz | 6.7 KB | ||
| Others |  emd_21132_additional.map.gz emd_21132_additional.map.gz emd_21132_half_map_1.map.gz emd_21132_half_map_1.map.gz emd_21132_half_map_2.map.gz emd_21132_half_map_2.map.gz | 96.9 MB 95.5 MB 95.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21132 http://ftp.pdbj.org/pub/emdb/structures/EMD-21132 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21132 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21132 | HTTPS FTP |
-Related structure data
| Related structure data |  6v9zMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10818 (Title: Cryo-electron microscopy reconstruction of PCAT1 bound to its CtA peptide substrate EMPIAR-10818 (Title: Cryo-electron microscopy reconstruction of PCAT1 bound to its CtA peptide substrateData size: 1.8 TB Data #1: Unaligned and uncorrected multiframe movies of PCAT1 bound to its CtA peptide substrate [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21132.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21132.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | full map from cryosparc | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.09 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: sharpened map (bfactor -75 Angstrom^2) from cryosparc
| File | emd_21132_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sharpened map (bfactor -75 Angstrom^2) from cryosparc | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map2 from cryosparc
| File | emd_21132_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map2 from cryosparc | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map1 from cryosparc
| File | emd_21132_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map1 from cryosparc | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Ternary complex of a homodimeric PCAT1 ABC transporter with two c...
| Entire | Name: Ternary complex of a homodimeric PCAT1 ABC transporter with two copies of bound peptide substrate |
|---|---|
| Components |
|
-Supramolecule #1: Ternary complex of a homodimeric PCAT1 ABC transporter with two c...
| Supramolecule | Name: Ternary complex of a homodimeric PCAT1 ABC transporter with two copies of bound peptide substrate type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Hungateiclostridium thermocellum (bacteria) Hungateiclostridium thermocellum (bacteria) |
| Molecular weight | Theoretical: 182.558 KDa |
-Macromolecule #1: ABC-type bacteriocin transporter
| Macromolecule | Name: ABC-type bacteriocin transporter / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Hungateiclostridium thermocellum (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) (bacteria) Hungateiclostridium thermocellum (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) (bacteria)Strain: ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372 |
| Molecular weight | Theoretical: 81.148742 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SNAMLRRLFK KKYVCVRQYD LTDAGAACLS SIAQYYGLKM SLAKIREMTG TDTQGTNAYG LIHAAKQLGF SAKGVKASKE DLLKDFRLP AIANVIVDNR LAHFVVIYSI KNRIITVADP GKGIVRYSMD DFCSIWTGGL VLLEPGEAFQ KGDYTQNMMV K FAGFLKPL ...String: SNAMLRRLFK KKYVCVRQYD LTDAGAACLS SIAQYYGLKM SLAKIREMTG TDTQGTNAYG LIHAAKQLGF SAKGVKASKE DLLKDFRLP AIANVIVDNR LAHFVVIYSI KNRIITVADP GKGIVRYSMD DFCSIWTGGL VLLEPGEAFQ KGDYTQNMMV K FAGFLKPL KKTVLCIFLA SLLYTALGIA GSFYIKFLFD DLIKFEKLND LHIISAGFAV IFLLQIFLNY YRSILVTKLG MS IDKSIMM EYYSHVLKLP MNFFNSRKVG EIISRFMDAS KIRQAISGAT LTIMIDTIMA VIGGILLYIQ NSSLFFISFI IIL LYGIIV TVFNKPIQNA NRQIMEDNAK LTSALVESVK GIETIKSFGA EEQTEKSTRD KIETVMKSSF KEGMLYINLS SLTG IVAGL GGIVILWAGA YNVIKGNMSG GQLLAFNALL AYFLTPVKNL IDLQPLIQTA VVASNRLGEI LELATEKELR EDSDD FVIS LKGDIEFRNV DFRYGLRKPV LKNINLTIPK GKTVAIVGES GSGKTTLAKL LMNFYSPEKG DILINGHSIK NISLEL IRK KIAFVSQDVF IFSGTVKENL CLGNENVDMD EIIKAAKMAN AHDFIEKLPL KYDTFLNESG ANLSEGQKQR LAIARAL LK KPDILILDEA TSNLDSITEN HIKDAIYGLE DDVTVIIIAH RLSTIVNCDK IYLLKDGEIV ESGSHTELIA LKGCYFKM W KQTENTLAS UniProtKB: ABC-type bacteriocin transporter |
-Macromolecule #2: CtA
| Macromolecule | Name: CtA / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Hungateiclostridium thermocellum (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) (bacteria) Hungateiclostridium thermocellum (strain ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372) (bacteria)Strain: ATCC 27405 / DSM 1237 / JCM 9322 / NBRC 103400 / NCIMB 10682 / NRRL B-4536 / VPI 7372 |
| Molecular weight | Theoretical: 10.217867 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SNAMSEAKKL NIGRELTDEE LMEMTGGSTF SIQCQKDYTY KPSLPVVKYG VVIDEPEVVI KYGVGPIVGI KYGVEPIGPI QPMYGIKPV ETLK UniProtKB: Bacteriocin-type signal sequence-containing protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7 Component:
| ||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: AIR | ||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 100.0 K |
| Specialist optics | Spherical aberration corrector: Microscope was modified with a Cs corrector Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-60 / Number grids imaged: 2 / Number real images: 3478 / Average exposure time: 0.2 sec. / Average electron dose: 1.33 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 0.0 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.7000000000000001 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)