+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20903 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
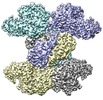
| Title | Structure of ACLY in the presence of citrate and CoA | |||||||||
 Map data Map data | ACLY in the presence of citrate and CoA | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | enzyme / LYASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationATP citrate synthase / ATP citrate synthase activity / citrate metabolic process / Fatty acyl-CoA biosynthesis / acetyl-CoA biosynthetic process / ChREBP activates metabolic gene expression / coenzyme A metabolic process / oxaloacetate metabolic process / negative regulation of ferroptosis / cholesterol biosynthetic process ...ATP citrate synthase / ATP citrate synthase activity / citrate metabolic process / Fatty acyl-CoA biosynthesis / acetyl-CoA biosynthetic process / ChREBP activates metabolic gene expression / coenzyme A metabolic process / oxaloacetate metabolic process / negative regulation of ferroptosis / cholesterol biosynthetic process / lipid biosynthetic process / fatty acid biosynthetic process / azurophil granule lumen / ficolin-1-rich granule lumen / ciliary basal body / Neutrophil degranulation / extracellular exosome / extracellular region / nucleoplasm / ATP binding / metal ion binding / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Xuepeng W / Ronen M | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2020 Journal: Nat Struct Mol Biol / Year: 2020Title: Molecular basis for acetyl-CoA production by ATP-citrate lyase. Authors: Xuepeng Wei / Kollin Schultz / Gleb A Bazilevsky / Austin Vogt / Ronen Marmorstein /  Abstract: ATP-citrate lyase (ACLY) synthesizes cytosolic acetyl coenzyme A (acetyl-CoA), a fundamental cellular building block. Accordingly, aberrant ACLY activity is observed in many diseases. Here we report ...ATP-citrate lyase (ACLY) synthesizes cytosolic acetyl coenzyme A (acetyl-CoA), a fundamental cellular building block. Accordingly, aberrant ACLY activity is observed in many diseases. Here we report cryo-EM structures of human ACLY, alone or bound to substrates or products. ACLY forms a homotetramer with a rigid citrate synthase homology (CSH) module, flanked by four flexible acetyl-CoA synthetase homology (ASH) domains; CoA is bound at the CSH-ASH interface in mutually exclusive productive or unproductive conformations. The structure of a catalytic mutant of ACLY in the presence of ATP, citrate and CoA substrates reveals a phospho-citryl-CoA intermediate in the ASH domain. ACLY with acetyl-CoA and oxaloacetate products shows the products bound in the ASH domain, with an additional oxaloacetate in the CSH domain, which could function in ACLY autoinhibition. These structures, which are supported by biochemical and biophysical data, challenge previous proposals of the ACLY catalytic mechanism and suggest additional therapeutic possibilities for ACLY-associated metabolic disorders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20903.map.gz emd_20903.map.gz | 14 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20903-v30.xml emd-20903-v30.xml emd-20903.xml emd-20903.xml | 13 KB 13 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20903.png emd_20903.png | 279.9 KB | ||
| Filedesc metadata |  emd-20903.cif.gz emd-20903.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20903 http://ftp.pdbj.org/pub/emdb/structures/EMD-20903 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20903 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20903 | HTTPS FTP |
-Related structure data
| Related structure data |  6uuzMC  6poeC  6pofC  6ui9C  6uiaC  6uuwC  6uv5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20903.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20903.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ACLY in the presence of citrate and CoA | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.87 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : ACLY, citrate and CoA
| Entire | Name: ACLY, citrate and CoA |
|---|---|
| Components |
|
-Supramolecule #1: ACLY, citrate and CoA
| Supramolecule | Name: ACLY, citrate and CoA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Location in cell: cytoplasm Homo sapiens (human) / Location in cell: cytoplasm |
| Molecular weight | Theoretical: 480 KDa |
-Macromolecule #1: ATP-citrate synthase
| Macromolecule | Name: ATP-citrate synthase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: ATP citrate synthase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 120.852938 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSAKAISEQT GKELLYKFIC TTSAIQNRFK YARVTPDTDW ARLLQDHPWL LSQNLVVKPD QLIKRRGKLG LVGVNLTLDG VKSWLKPRL GQEATVGKAT GFLKNFLIEP FVPHSQAEEF YVCIYATREG DYVLFHHEGG VDVGDVDAKA QKLLVGVDEK L NPEDIKKH ...String: MSAKAISEQT GKELLYKFIC TTSAIQNRFK YARVTPDTDW ARLLQDHPWL LSQNLVVKPD QLIKRRGKLG LVGVNLTLDG VKSWLKPRL GQEATVGKAT GFLKNFLIEP FVPHSQAEEF YVCIYATREG DYVLFHHEGG VDVGDVDAKA QKLLVGVDEK L NPEDIKKH LLVHAPEDKK EILASFISGL FNFYEDLYFT YLEINPLVVT KDGVYVLDLA AKVDATADYI CKVKWGDIEF PP PFGREAY PEEAYIADLD AKSGASLKLT LLNPKGRIWT MVAGGGASVV YSDTICDLGG VNELANYGEY SGAPSEQQTY DYA KTILSL MTREKHPDGK ILIIGGSIAN FTNVAATFKG IVRAIRDYQG PLKEHEVTIF VRRGGPNYQE GLRVMGEVGK TTGI PIHVF GTETHMTAIV GMALGHRPIP NQPPTAAHTA NFLLNASGST STPAPSRTAS FSESRADEVA PAKKAKPAMP QDSVP SPRS LQGKSTTLFS RHTKAIVWGM QTRAVQGMLD FDYVCSRDEP SVAAMVYPFT GDHKQKFYWG HKEILIPVFK NMADAM RKH PEVDVLINFA SLRSAYDSTM ETMNYAQIRT IAIIAEGIPE ALTRKLIKKA DQKGVTIIGP ATVGGIKPGC FKIGNTG GM LDNILASKLY RPGSVAYVSR SGGMSNELNN IISRTTDGVY EGVAIGGDRY PGSTFMDHVL RYQDTPGVKM IVVLGEIG G TEEYKICRGI KEGRLTKPIV CWCIGTCATM FSSEVQFGHA GACANQASET AVAKNQALKE AGVFVPRSFD ELGEIIQSV YEDLVANGVI VPAQEVPPPT VPMDYSWARE LGLIRKPASF MTSICDERGQ ELIYAGMPIT EVFKEEMGIG GVLGLLWFQK RLPKYSCQF IEMCLMVTAD HGPAVSGAHN TIICARAGKD LVSSLTSGLL TIGDRFGGAL DAAAKMFSKA FDSGIIPMEF V NKMKKEGK LIMGIGHRVK SINNPDMRVQ ILKDYVRQHF PATPLLDYAL EVEKITTSKK PNLILNVDGL IGVAFVDMLR NC GSFTREE ADEYIDIGAL NGIFVLGRSM GFIGHYLDQK RLKQGLYRHP WDDISYVLPE HMS UniProtKB: ATP-citrate synthase |
-Macromolecule #2: COENZYME A
| Macromolecule | Name: COENZYME A / type: ligand / ID: 2 / Number of copies: 4 / Formula: COA |
|---|---|
| Molecular weight | Theoretical: 767.534 Da |
| Chemical component information |  ChemComp-COA: |
-Macromolecule #3: water
| Macromolecule | Name: water / type: ligand / ID: 3 / Number of copies: 2 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Component - Concentration: 200.0 mM / Component - Formula: NaCl / Component - Name: Sodium Chloride |
| Grid | Support film - Material: CARBON / Support film - topology: HOLEY / Details: unspecified |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 289 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 3624 / Average exposure time: 1.3 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















