+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20788 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human CALHM2 in an active/open state | |||||||||
 Map data Map data | Final map refined with a soft solvent mask. Improved resolution for the well-defined parts of the protein, yet incomplete densities for the highly flexible regions such as S1. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | calcium homeostasis modulator / CALHM2 / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of microglial cell activation / ATP export / calcium ion import / monoatomic cation channel activity / regulation of synaptic plasticity / positive regulation of apoptotic process / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Lu W / Du J | |||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: The structures and gating mechanism of human calcium homeostasis modulator 2. Authors: Wooyoung Choi / Nicolina Clemente / Weinan Sun / Juan Du / Wei Lü /  Abstract: Calcium homeostasis modulators (CALHMs) are voltage-gated, Ca-inhibited nonselective ion channels that act as major ATP release channels, and have important roles in gustatory signalling and neuronal ...Calcium homeostasis modulators (CALHMs) are voltage-gated, Ca-inhibited nonselective ion channels that act as major ATP release channels, and have important roles in gustatory signalling and neuronal toxicity. Dysfunction of CALHMs has previously been linked to neurological disorders. Here we present cryo-electron microscopy structures of the human CALHM2 channel in the Ca-free active or open state and in the ruthenium red (RUR)-bound inhibited state, at resolutions up to 2.7 Å. Our work shows that purified CALHM2 channels form both gap junctions and undecameric hemichannels. The protomer shows a mirrored arrangement of the transmembrane domains (helices S1-S4) relative to other channels with a similar topology, such as connexins, innexins and volume-regulated anion channels. Upon binding to RUR, we observed a contracted pore with notable conformational changes of the pore-lining helix S1, which swings nearly 60° towards the pore axis from a vertical to a lifted position. We propose a two-section gating mechanism in which the S1 helix coarsely adjusts, and the N-terminal helix fine-tunes, the pore size. We identified a RUR-binding site near helix S1 that may stabilize this helix in the lifted conformation, giving rise to channel inhibition. Our work elaborates on the principles of CALHM2 channel architecture and symmetry, and the mechanism that underlies channel inhibition. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20788.map.gz emd_20788.map.gz | 117.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20788-v30.xml emd-20788-v30.xml emd-20788.xml emd-20788.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20788.png emd_20788.png | 211.2 KB | ||
| Masks |  emd_20788_msk_1.map emd_20788_msk_1.map | 149.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-20788.cif.gz emd-20788.cif.gz | 5.4 KB | ||
| Others |  emd_20788_additional_1.map.gz emd_20788_additional_1.map.gz emd_20788_additional_2.map.gz emd_20788_additional_2.map.gz | 117.1 MB 423.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20788 http://ftp.pdbj.org/pub/emdb/structures/EMD-20788 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20788 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20788 | HTTPS FTP |
-Validation report
| Summary document |  emd_20788_validation.pdf.gz emd_20788_validation.pdf.gz | 493.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_20788_full_validation.pdf.gz emd_20788_full_validation.pdf.gz | 493.2 KB | Display | |
| Data in XML |  emd_20788_validation.xml.gz emd_20788_validation.xml.gz | 6.7 KB | Display | |
| Data in CIF |  emd_20788_validation.cif.gz emd_20788_validation.cif.gz | 7.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20788 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20788 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20788 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-20788 | HTTPS FTP |
-Related structure data
| Related structure data |  6uivMC  6uiwC  6uixC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_20788.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20788.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final map refined with a soft solvent mask. Improved resolution for the well-defined parts of the protein, yet incomplete densities for the highly flexible regions such as S1. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.026 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
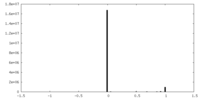
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_20788_msk_1.map emd_20788_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
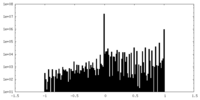
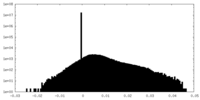
| Density Histograms |
-Additional map: Map refined without a soft solvent mask. Better...
| File | emd_20788_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map refined without a soft solvent mask. Better visualization of the highly flexible regions such as S1. | ||||||||||||
| Projections & Slices |
| ||||||||||||
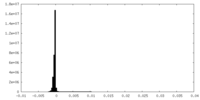
| Density Histograms |
-Additional map: Map of single subunit with vertical S1. This...
| File | emd_20788_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map of single subunit with vertical S1. This map is obtained through symmetry expansion and signal subtraction of single subunit, followed by 3D classification. | ||||||||||||
| Projections & Slices |
| ||||||||||||
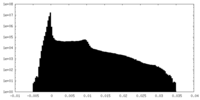
| Density Histograms |
- Sample components
Sample components
-Entire : human CALHM2
| Entire | Name: human CALHM2 |
|---|---|
| Components |
|
-Supramolecule #1: human CALHM2
| Supramolecule | Name: human CALHM2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Calcium homeostasis modulator protein 2
| Macromolecule | Name: Calcium homeostasis modulator protein 2 / type: protein_or_peptide / ID: 1 / Number of copies: 11 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.198676 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAALIAENFR FLSLFFKSKD VMIFNGLVAL GTVGSQELFS VVAFHCPCSP ARNYLYGLAA IGVPALVLFI IGIILNNHTW NLVAECQHR RTKNCSAAPT FLLLSSILGR AAVAPVTWSV ISLLRGEAYV CALSEFVDPS SLTAREEHFP SAHATEILAR F PCKENPDN ...String: MAALIAENFR FLSLFFKSKD VMIFNGLVAL GTVGSQELFS VVAFHCPCSP ARNYLYGLAA IGVPALVLFI IGIILNNHTW NLVAECQHR RTKNCSAAPT FLLLSSILGR AAVAPVTWSV ISLLRGEAYV CALSEFVDPS SLTAREEHFP SAHATEILAR F PCKENPDN LSDFREEVSR RLRYESQLFG WLLIGVVAIL VFLTKCLKHY CSPLSYRQEA YWAQYRANED QLFQRTAEVH SR VLAANNV RRFFGFVALN KDDEELIANF PVEGTQPRPQ WNAITGVYLY RENQGLPLYS RLHKWAQGLA GNGAAPDNVE MAL LPSFES RLVPR UniProtKB: Calcium homeostasis modulator protein 2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average exposure time: 8.0 sec. / Average electron dose: 54.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-6uiv: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)