+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0920 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the human CALHM2 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | channel / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of microglial cell activation / ATP export / calcium ion import / monoatomic cation channel activity / regulation of synaptic plasticity / positive regulation of apoptotic process / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
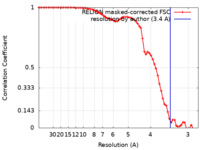
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Demura K / Kusakizako T | |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2020 Journal: Sci Adv / Year: 2020Title: Cryo-EM structures of calcium homeostasis modulator channels in diverse oligomeric assemblies. Authors: Kanae Demura / Tsukasa Kusakizako / Wataru Shihoya / Masahiro Hiraizumi / Kengo Nomura / Hiroto Shimada / Keitaro Yamashita / Tomohiro Nishizawa / Akiyuki Taruno / Osamu Nureki /  Abstract: Calcium homeostasis modulator (CALHM) family proteins are Ca-regulated adenosine triphosphate (ATP)-release channels involved in neural functions including neurotransmission in gustation. Here, we ...Calcium homeostasis modulator (CALHM) family proteins are Ca-regulated adenosine triphosphate (ATP)-release channels involved in neural functions including neurotransmission in gustation. Here, we present the cryo-electron microscopy (EM) structures of killifish CALHM1, human CALHM2, and CLHM-1 at resolutions of 2.66, 3.4, and 3.6 Å, respectively. The CALHM1 octamer structure reveals that the N-terminal helix forms the constriction site at the channel pore in the open state and modulates the ATP conductance. The CALHM2 undecamer and CLHM-1 nonamer structures show the different oligomeric stoichiometries among CALHM homologs. We further report the cryo-EM structures of the chimeric construct, revealing that the intersubunit interactions at the transmembrane domain (TMD) and the TMD-intracellular domain linker define the oligomeric stoichiometry. These findings advance our understanding of the ATP conduction and oligomerization mechanisms of CALHM channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0920.map.gz emd_0920.map.gz | 3.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0920-v30.xml emd-0920-v30.xml emd-0920.xml emd-0920.xml | 16.6 KB 16.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0920_fsc.xml emd_0920_fsc.xml | 6.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_0920.png emd_0920.png | 141.2 KB | ||
| Masks |  emd_0920_msk_1.map emd_0920_msk_1.map | 26.2 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-0920.cif.gz emd-0920.cif.gz | 5.8 KB | ||
| Others |  emd_0920_half_map_1.map.gz emd_0920_half_map_1.map.gz emd_0920_half_map_2.map.gz emd_0920_half_map_2.map.gz | 19.9 MB 19.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0920 http://ftp.pdbj.org/pub/emdb/structures/EMD-0920 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0920 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0920 | HTTPS FTP |
-Related structure data
| Related structure data |  6lmuMC  0919C  0921C  0922C  0923C  6lmtC  6lmvC  6lmwC  6lmxC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10444 (Title: Cryo-EM structures of calcium homeostasis modulator (CALHM) channels EMPIAR-10444 (Title: Cryo-EM structures of calcium homeostasis modulator (CALHM) channelsData size: 6.8 TB Data #1: Unaligned movies for OlCALHM1 [micrographs - multiframe] Data #2: Unaligned movies for HsCALHM2 [micrographs - multiframe] Data #3: Unaligned movies for CeCLHM-1 [micrographs - multiframe] Data #4: Unaligned movies for OlCALHM1-HsCALHM2 chimera [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0920.map.gz / Format: CCP4 / Size: 26.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0920.map.gz / Format: CCP4 / Size: 26.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.36172 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_0920_msk_1.map emd_0920_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_0920_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_0920_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CALHM2
| Entire | Name: CALHM2 |
|---|---|
| Components |
|
-Supramolecule #1: CALHM2
| Supramolecule | Name: CALHM2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Calcium homeostasis modulator protein 2
| Macromolecule | Name: Calcium homeostasis modulator protein 2 / type: protein_or_peptide / ID: 1 / Number of copies: 11 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.006367 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAALIAENFR FLSLFFKSKD VMIFNGLVAL GTVGSQELFS VVAFHCPCSP ARNYLYGLAA IGVPALVLFI IGIILNNHTW NLVAECQHR RTKNCSAAPT FLLLSSILGR AAVAPVTWSV ISLLRGEAYV CALSEFVDPS SLTAREEHFP SAHATEILAR F PCKENPDN ...String: MAALIAENFR FLSLFFKSKD VMIFNGLVAL GTVGSQELFS VVAFHCPCSP ARNYLYGLAA IGVPALVLFI IGIILNNHTW NLVAECQHR RTKNCSAAPT FLLLSSILGR AAVAPVTWSV ISLLRGEAYV CALSEFVDPS SLTAREEHFP SAHATEILAR F PCKENPDN LSDFREEVSR RLRYESQLFG WLLIGVVAIL VFLTKCLKHY CSPLSYRQEA YWAQYRANED QLFQRTAEVH SR VLAANNV RRFFGFVALN KDDEELIANF PVEGTQPRPQ WNAITGVYLY RENQGLPLYS RLHKWAQGLA GNGAAPDNVE MAL LPSENL YFQ UniProtKB: Calcium homeostasis modulator protein 2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)