+Search query
-Structure paper
| Title | The structures and gating mechanism of human calcium homeostasis modulator 2. |
|---|---|
| Journal, issue, pages | Nature, Vol. 576, Issue 7785, Page 163-167, Year 2019 |
| Publish date | Nov 27, 2019 |
 Authors Authors | Wooyoung Choi / Nicolina Clemente / Weinan Sun / Juan Du / Wei Lü /  |
| PubMed Abstract | Calcium homeostasis modulators (CALHMs) are voltage-gated, Ca-inhibited nonselective ion channels that act as major ATP release channels, and have important roles in gustatory signalling and neuronal ...Calcium homeostasis modulators (CALHMs) are voltage-gated, Ca-inhibited nonselective ion channels that act as major ATP release channels, and have important roles in gustatory signalling and neuronal toxicity. Dysfunction of CALHMs has previously been linked to neurological disorders. Here we present cryo-electron microscopy structures of the human CALHM2 channel in the Ca-free active or open state and in the ruthenium red (RUR)-bound inhibited state, at resolutions up to 2.7 Å. Our work shows that purified CALHM2 channels form both gap junctions and undecameric hemichannels. The protomer shows a mirrored arrangement of the transmembrane domains (helices S1-S4) relative to other channels with a similar topology, such as connexins, innexins and volume-regulated anion channels. Upon binding to RUR, we observed a contracted pore with notable conformational changes of the pore-lining helix S1, which swings nearly 60° towards the pore axis from a vertical to a lifted position. We propose a two-section gating mechanism in which the S1 helix coarsely adjusts, and the N-terminal helix fine-tunes, the pore size. We identified a RUR-binding site near helix S1 that may stabilize this helix in the lifted conformation, giving rise to channel inhibition. Our work elaborates on the principles of CALHM2 channel architecture and symmetry, and the mechanism that underlies channel inhibition. |
 External links External links |  Nature / Nature /  PubMed:31776515 / PubMed:31776515 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.7 - 3.5 Å |
| Structure data | EMDB-20788, PDB-6uiv: EMDB-20789, PDB-6uiw: EMDB-20790, PDB-6uix: |
| Chemicals | 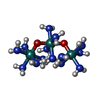 ChemComp-R2R: |
| Source |
|
 Keywords Keywords | TRANSPORT PROTEIN / calcium homeostasis modulator / CALHM2 / TRANSPORT PROTEIN/INHIBITOR / ruthenium red / TRANSPORT PROTEIN-INHIBITOR complex / gap junction |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers









 homo sapiens (human)
homo sapiens (human)