[English] 日本語
 Yorodumi
Yorodumi- EMDB-19743: RNA polymerase II core initially transcribing complex with an ord... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
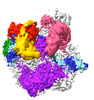
| Title | RNA polymerase II core initially transcribing complex with an ordered RNA of 12 nt | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RNA polymerase II / promoter escape / de novo transcription / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of core promoter binding / RNA polymerase II core complex assembly / transcription factor TFIIE complex / RNA polymerase transcription factor SL1 complex / meiotic sister chromatid cohesion / phosphatase activator activity / RNA polymerase III general transcription initiation factor activity / TFIIF-class transcription factor complex binding / transcriptional start site selection at RNA polymerase II promoter / RNA polymerase I core promoter sequence-specific DNA binding ...positive regulation of core promoter binding / RNA polymerase II core complex assembly / transcription factor TFIIE complex / RNA polymerase transcription factor SL1 complex / meiotic sister chromatid cohesion / phosphatase activator activity / RNA polymerase III general transcription initiation factor activity / TFIIF-class transcription factor complex binding / transcriptional start site selection at RNA polymerase II promoter / RNA polymerase I core promoter sequence-specific DNA binding / transcription factor TFIIF complex / RNA Polymerase III Transcription Initiation From Type 1 Promoter / RNA Polymerase III Transcription Initiation From Type 2 Promoter / RNA Polymerase III Transcription Initiation From Type 3 Promoter / Formation of RNA Pol II elongation complex / Formation of the Early Elongation Complex / Transcriptional regulation by small RNAs / RNA Polymerase II Pre-transcription Events / TP53 Regulates Transcription of DNA Repair Genes / FGFR2 alternative splicing / RNA polymerase II transcribes snRNA genes / mRNA Capping / mRNA Splicing - Minor Pathway / Processing of Capped Intron-Containing Pre-mRNA / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Elongation / RNA Polymerase II Transcription Initiation And Promoter Clearance / RNA Pol II CTD phosphorylation and interaction with CE / Estrogen-dependent gene expression / Formation of TC-NER Pre-Incision Complex / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / mRNA Splicing - Major Pathway / transcription factor TFIIA complex / female germ cell nucleus / RNA Polymerase III Abortive And Retractive Initiation / male pronucleus / female pronucleus / germinal vesicle / RNA polymerase II general transcription initiation factor binding / nuclear thyroid hormone receptor binding / Abortive elongation of HIV-1 transcript in the absence of Tat / transcription preinitiation complex / FGFR2 alternative splicing / RNA Polymerase I Transcription Termination / Viral Messenger RNA Synthesis / Signaling by FGFR2 IIIa TM / protein acetylation / RNA polymerase II general transcription initiation factor activity / transcription factor TFIID complex / cell division site / acetyltransferase activity / RNA Pol II CTD phosphorylation and interaction with CE during HIV infection / RNA Pol II CTD phosphorylation and interaction with CE / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / Formation of the Early Elongation Complex / Formation of the HIV-1 Early Elongation Complex / mRNA Capping / mRNA Splicing - Minor Pathway / aryl hydrocarbon receptor binding / viral transcription / TFIIB-class transcription factor binding / RNA polymerase II complex binding / RNA Polymerase I Transcription Initiation / maintenance of transcriptional fidelity during transcription elongation by RNA polymerase II / Processing of Capped Intron-Containing Pre-mRNA / transcription by RNA polymerase III / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / RNA polymerase II transcribes snRNA genes / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / positive regulation of transcription initiation by RNA polymerase II / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / histone acetyltransferase activity / RNA polymerase I complex / transcription elongation by RNA polymerase I / core promoter sequence-specific DNA binding / RNA polymerase III complex / RNA polymerase II core promoter sequence-specific DNA binding / Formation of HIV elongation complex in the absence of HIV Tat / RNA polymerase II preinitiation complex assembly / histone acetyltransferase / tRNA transcription by RNA polymerase III / RNA polymerase II, core complex / RNA Polymerase II Transcription Elongation / negative regulation of protein binding / Formation of RNA Pol II elongation complex / spindle assembly / transcription-coupled nucleotide-excision repair / translation initiation factor binding Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /  unidentified adenovirus unidentified adenovirus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Zhan Y / Grabbe F / Oberbeckmann E / Dienemann C / Cramer P | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2024 Journal: Mol Cell / Year: 2024Title: Three-step mechanism of promoter escape by RNA polymerase II. Authors: Yumeng Zhan / Frauke Grabbe / Elisa Oberbeckmann / Christian Dienemann / Patrick Cramer /  Abstract: The transition from transcription initiation to elongation is highly regulated in human cells but remains incompletely understood at the structural level. In particular, it is unclear how ...The transition from transcription initiation to elongation is highly regulated in human cells but remains incompletely understood at the structural level. In particular, it is unclear how interactions between RNA polymerase II (RNA Pol II) and initiation factors are broken to enable promoter escape. Here, we reconstitute RNA Pol II promoter escape in vitro and determine high-resolution structures of initially transcribing complexes containing 8-, 10-, and 12-nt ordered RNAs and two elongation complexes containing 14-nt RNAs. We suggest that promoter escape occurs in three major steps. First, the growing RNA displaces the B-reader element of the initiation factor TFIIB without evicting TFIIB. Second, the rewinding of the transcription bubble coincides with the eviction of TFIIA, TFIIB, and TBP. Third, the binding of DSIF and NELF facilitates TFIIE and TFIIH dissociation, establishing the paused elongation complex. This three-step model for promoter escape fills a gap in our understanding of the initiation-elongation transition of RNA Pol II transcription. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19743.map.gz emd_19743.map.gz | 213.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19743-v30.xml emd-19743-v30.xml emd-19743.xml emd-19743.xml | 43.9 KB 43.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_19743.png emd_19743.png | 163.4 KB | ||
| Masks |  emd_19743_msk_1.map emd_19743_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-19743.cif.gz emd-19743.cif.gz | 11.7 KB | ||
| Others |  emd_19743_half_map_1.map.gz emd_19743_half_map_1.map.gz emd_19743_half_map_2.map.gz emd_19743_half_map_2.map.gz | 194.2 MB 194.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19743 http://ftp.pdbj.org/pub/emdb/structures/EMD-19743 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19743 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19743 | HTTPS FTP |
-Related structure data
| Related structure data |  8s5nMC  8s51C  8s52C  8s54C  8s55C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19743.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19743.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||
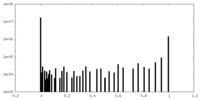
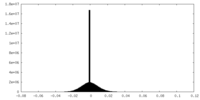
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_19743_msk_1.map emd_19743_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
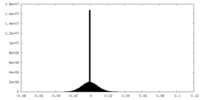
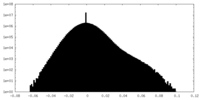
| Density Histograms |
-Half map: #2
| File | emd_19743_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
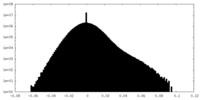
| Density Histograms |
-Half map: #1
| File | emd_19743_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Core initially transcribing complex with and ordered RNA of 12 nt
+Supramolecule #1: Core initially transcribing complex with and ordered RNA of 12 nt
+Supramolecule #2: RNA polymerase II
+Macromolecule #1: DNA-directed RNA polymerase subunit
+Macromolecule #2: DNA-directed RNA polymerase subunit beta
+Macromolecule #3: DNA-directed RNA polymerase II subunit RPB3
+Macromolecule #4: RNA polymerase II subunit D
+Macromolecule #5: DNA-directed RNA polymerase II subunit E
+Macromolecule #6: DNA-directed RNA polymerases I, II, and III subunit RPABC2
+Macromolecule #7: DNA-directed RNA polymerase II subunit RPB7
+Macromolecule #8: DNA-directed RNA polymerases I, II, and III subunit RPABC3
+Macromolecule #9: DNA-directed RNA polymerase II subunit RPB9
+Macromolecule #10: DNA-directed RNA polymerases I, II, and III subunit RPABC5
+Macromolecule #11: DNA-directed RNA polymerase II subunit RPB11-a
+Macromolecule #12: RNA polymerase II subunit K
+Macromolecule #13: Transcription initiation factor IIB
+Macromolecule #15: TATA-box-binding protein
+Macromolecule #17: General transcription factor IIF subunit 1
+Macromolecule #18: General transcription factor IIF subunit 2
+Macromolecule #20: Transcription initiation factor IIA subunit 1
+Macromolecule #21: Transcription initiation factor IIA subunit 2
+Macromolecule #22: General transcription factor IIE subunit 1
+Macromolecule #23: Transcription initiation factor IIE subunit beta
+Macromolecule #14: Non-template DNA
+Macromolecule #19: Template DNA
+Macromolecule #16: RNA
+Macromolecule #24: ZINC ION
+Macromolecule #25: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.7 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 18827 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller


































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)








































 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)
