+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | H. sapiens MCM bound to double stranded DNA and ORC1-6 | |||||||||||||||
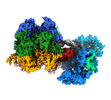
 マップデータ マップデータ | Composite map of the H. sapiens MCM-ORC complex bound to DNA | |||||||||||||||
 試料 試料 |
| |||||||||||||||
 キーワード キーワード | AAA+ ATPase / DNA helicase / REPLICATION | |||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報polar body extrusion after meiotic divisions / CDC6 association with the ORC:origin complex / origin recognition complex / E2F-enabled inhibition of pre-replication complex formation / Switching of origins to a post-replicative state / Unwinding of DNA / nuclear origin of replication recognition complex / Regulation of MITF-M-dependent genes involved in DNA replication, damage repair and senescence / alpha DNA polymerase:primase complex / mitotic DNA replication ...polar body extrusion after meiotic divisions / CDC6 association with the ORC:origin complex / origin recognition complex / E2F-enabled inhibition of pre-replication complex formation / Switching of origins to a post-replicative state / Unwinding of DNA / nuclear origin of replication recognition complex / Regulation of MITF-M-dependent genes involved in DNA replication, damage repair and senescence / alpha DNA polymerase:primase complex / mitotic DNA replication / CMG complex / inner kinetochore / regulation of phosphorylation / nuclear pre-replicative complex / DNA replication preinitiation complex / MCM complex / mitotic DNA replication checkpoint signaling / double-strand break repair via break-induced replication / mitotic DNA replication initiation / neural precursor cell proliferation / regulation of DNA-templated DNA replication initiation / DNA strand elongation involved in DNA replication / cochlea development / G1/S-Specific Transcription / regulation of DNA replication / DNA replication origin binding / protein polymerization / Activation of the pre-replicative complex / DNA replication initiation / glial cell proliferation / heterochromatin / Activation of ATR in response to replication stress / cellular response to epidermal growth factor stimulus / Assembly of the ORC complex at the origin of replication / cellular response to interleukin-4 / Assembly of the pre-replicative complex / fibrillar center / Orc1 removal from chromatin / cellular response to xenobiotic stimulus / nucleosome assembly / single-stranded DNA binding / DNA helicase / histone binding / forked DNA-dependent helicase activity / single-stranded 3'-5' DNA helicase activity / four-way junction helicase activity / double-stranded DNA helicase activity / chromosome, telomeric region / cell population proliferation / DNA replication / nuclear body / cilium / nucleotide binding / intracellular membrane-bounded organelle / apoptotic process / centrosome / DNA damage response / chromatin binding / chromatin / nucleolus / perinuclear region of cytoplasm / enzyme binding / negative regulation of transcription by RNA polymerase II / ATP hydrolysis activity / DNA binding / zinc ion binding / nucleoplasm / ATP binding / metal ion binding / identical protein binding / nucleus / membrane / cytosol / cytoplasm 類似検索 - 分子機能 | |||||||||||||||
| 生物種 | synthetic construct (人工物) /  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.6 Å | |||||||||||||||
 データ登録者 データ登録者 | Greiwe JF / Weissmann F / Diffley JFX / Costa A | |||||||||||||||
| 資金援助 |  英国, European Union, 4件 英国, European Union, 4件
| |||||||||||||||
 引用 引用 | ジャーナル: Acta Crystallogr D Struct Biol / 年: 2019 タイトル: Macromolecular structure determination using X-rays, neutrons and electrons: recent developments in Phenix. 著者: Dorothee Liebschner / Pavel V Afonine / Matthew L Baker / Gábor Bunkóczi / Vincent B Chen / Tristan I Croll / Bradley Hintze / Li Wei Hung / Swati Jain / Airlie J McCoy / Nigel W Moriarty / ...著者: Dorothee Liebschner / Pavel V Afonine / Matthew L Baker / Gábor Bunkóczi / Vincent B Chen / Tristan I Croll / Bradley Hintze / Li Wei Hung / Swati Jain / Airlie J McCoy / Nigel W Moriarty / Robert D Oeffner / Billy K Poon / Michael G Prisant / Randy J Read / Jane S Richardson / David C Richardson / Massimo D Sammito / Oleg V Sobolev / Duncan H Stockwell / Thomas C Terwilliger / Alexandre G Urzhumtsev / Lizbeth L Videau / Christopher J Williams / Paul D Adams /    要旨: Diffraction (X-ray, neutron and electron) and electron cryo-microscopy are powerful methods to determine three-dimensional macromolecular structures, which are required to understand biological ...Diffraction (X-ray, neutron and electron) and electron cryo-microscopy are powerful methods to determine three-dimensional macromolecular structures, which are required to understand biological processes and to develop new therapeutics against diseases. The overall structure-solution workflow is similar for these techniques, but nuances exist because the properties of the reduced experimental data are different. Software tools for structure determination should therefore be tailored for each method. Phenix is a comprehensive software package for macromolecular structure determination that handles data from any of these techniques. Tasks performed with Phenix include data-quality assessment, map improvement, model building, the validation/rebuilding/refinement cycle and deposition. Each tool caters to the type of experimental data. The design of Phenix emphasizes the automation of procedures, where possible, to minimize repetitive and time-consuming manual tasks, while default parameters are chosen to encourage best practice. A graphical user interface provides access to many command-line features of Phenix and streamlines the transition between programs, project tracking and re-running of previous tasks. #1:  ジャーナル: Biorxiv / 年: 2024 ジャーナル: Biorxiv / 年: 2024タイトル: MCM Double Hexamer Loading Visualised with Human Proteins 著者: Weissmann F / Greiwe JF / Puhringer T / Miller TCR / Diffley JFX / Costa A | |||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_19622.map.gz emd_19622.map.gz | 211.9 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-19622-v30.xml emd-19622-v30.xml emd-19622.xml emd-19622.xml | 44.7 KB 44.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_19622.png emd_19622.png | 119.4 KB | ||
| Filedesc metadata |  emd-19622.cif.gz emd-19622.cif.gz | 13.4 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19622 http://ftp.pdbj.org/pub/emdb/structures/EMD-19622 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19622 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19622 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  8s0dMC  8s09C  8s0aC  8s0bC  8s0cC  8s0eC  8s0fC C: 同じ文献を引用 ( M: このマップから作成された原子モデル |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_19622.map.gz / 形式: CCP4 / 大きさ: 244.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_19622.map.gz / 形式: CCP4 / 大きさ: 244.1 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Composite map of the H. sapiens MCM-ORC complex bound to DNA | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
- 試料の構成要素
試料の構成要素
+全体 : H. sapiens MCM bound to double stranded DNA and ORC1-6
+超分子 #1: H. sapiens MCM bound to double stranded DNA and ORC1-6
+超分子 #2: Double stranded DNA
+超分子 #3: H. sapiens MCM
+超分子 #4: H. sapiens ORC1-5
+超分子 #5: H. sapiens ORC6
+分子 #1: Origin recognition complex subunit 6
+分子 #2: DNA replication licensing factor MCM2
+分子 #3: DNA replication licensing factor MCM3
+分子 #4: DNA replication licensing factor MCM4
+分子 #5: DNA replication licensing factor MCM5
+分子 #6: DNA replication licensing factor MCM6
+分子 #7: DNA replication licensing factor MCM7
+分子 #10: Origin recognition complex subunit 1
+分子 #11: Origin recognition complex subunit 2
+分子 #12: Isoform 2 of Origin recognition complex subunit 3
+分子 #13: Origin recognition complex subunit 4
+分子 #14: Origin recognition complex subunit 5
+分子 #8: DNA (58-mer)
+分子 #9: DNA (58-mer)
+分子 #15: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
+分子 #16: MAGNESIUM ION
+分子 #17: ZINC ION
+分子 #18: ADENOSINE-5'-DIPHOSPHATE
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 |
|---|---|
| グリッド | モデル: UltrAuFoil R1.2/1.3 / 材質: GOLD / メッシュ: 300 / 支持フィルム - 材質: GRAPHENE OXIDE |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 90 % / チャンバー内温度: 295 K / 装置: FEI VITROBOT MARK IV |
| 詳細 | The MCM recruitment reaction was reconstituted in vitro using purified H. sapiens proteins, a short DNA template and ATPgammaS. Four microlitres of the entire reaction was applied on a grid and incubated for 1 min at room temperature before blotting with filter paper for 5 s and plunge-freezing in liquid ethane. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 特殊光学系 | エネルギーフィルター - 名称: GIF Bioquantum |
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 検出モード: COUNTING / 撮影したグリッド数: 1 / 実像数: 31569 / 平均露光時間: 9.4 sec. / 平均電子線量: 49.28 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 2.5 µm / 最小 デフォーカス(公称値): 1.0 µm / 倍率(公称値): 130000 |
| 試料ステージ | ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: NONE |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.6 Å / 解像度の算出法: FSC 0.143 CUT-OFF / ソフトウェア - 名称: cryoSPARC (ver. 3.3.2) 詳細: The here represented map is a composite map of the consensus refinement and the locally refined map encompassing the ORC1-5 complex. 使用した粒子像数: 182341 |
| 初期 角度割当 | タイプ: MAXIMUM LIKELIHOOD / ソフトウェア - 名称: cryoSPARC (ver. 3.3.2) |
| 最終 角度割当 | タイプ: MAXIMUM LIKELIHOOD / ソフトウェア - 名称: cryoSPARC (ver. 3.3.2) |
-原子モデル構築 1
| 詳細 | The models of MCM2-7-ORC6 and ORC1-5 that were refined against the consensus refinement of the MCM-ORC complex and the locally refined ORC1-5, respectively, were fitted into the composite map using rigid body docking. |
|---|---|
| 得られたモデル |  PDB-8s0d: |
 ムービー
ムービー コントローラー
コントローラー
















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)
























