[English] 日本語
 Yorodumi
Yorodumi- EMDB-1795: The three-dimensional reconstruction of the baseplate of TP901-1 ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1795 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
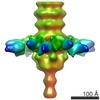
| Title | The three-dimensional reconstruction of the baseplate of TP901-1 bppU mutant | |||||||||
 Map data Map data | The three-dimensional representation of the baseplate of TP901-1 bppU mutant | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TP901-1 / bppU / Baseplate / Electron microscopy | |||||||||
| Biological species |  Lactococcus phage TP901-1 (virus) Lactococcus phage TP901-1 (virus) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 28.0 Å | |||||||||
 Authors Authors | Bebeacua C / Bron P / Lai L / SkovgaardVegge C / Brondsted L / Spinelli S / Campanacci V / Veesler D / vanHeel M / Cambillau C | |||||||||
 Citation Citation |  Journal: J Biol Chem / Year: 2010 Journal: J Biol Chem / Year: 2010Title: Structure and molecular assignment of lactococcal phage TP901-1 baseplate. Authors: Cecilia Bebeacua / Patrick Bron / Livia Lai / Christina Skovgaard Vegge / Lone Brøndsted / Silvia Spinelli / Valérie Campanacci / David Veesler / Marin van Heel / Christian Cambillau /  Abstract: P335 lactococcal phages infect the gram(+) bacterium Lactococcus lactis using a large multiprotein complex located at the distal part of the tail and termed baseplate (BP). The BP harbors the ...P335 lactococcal phages infect the gram(+) bacterium Lactococcus lactis using a large multiprotein complex located at the distal part of the tail and termed baseplate (BP). The BP harbors the receptor-binding proteins (RBPs), which allow the specific recognition of saccharidic receptors localized on the host cell surface. We report here the electron microscopic structure of the phage TP901-1 wild-type BP as well as those of two mutants bppL (-) and bppU(-), lacking BppL (the RBPs) or both peripheral BP components (BppL and BppU), respectively. We also achieved an electron microscopic reconstruction of a partial BP complex, formed by BppU and BppL. This complex exhibits a tripod shape and is composed of nine BppLs and three BppUs. These structures, combined with light-scattering measurements, led us to propose that the TP901-1 BP harbors six tripods at its periphery, located around the central tube formed by ORF46 (Dit) hexamers, at its proximal end, and a ORF47 (Tal) trimer at its distal extremity. A total of 54 BppLs (18 RBPs) are thus available to mediate host anchoring with a large apparent avidity. TP901-1 BP exhibits an infection-ready conformation and differs strikingly from the lactococcal phage p2 BP, bearing only 6 RBPs, and which needs a conformational change to reach its activated state. The comparison of several Siphoviridae structures uncovers a close organization of their central BP core whereas striking differences occur at the periphery, leading to diverse mechanisms of host recognition. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1795.map.gz emd_1795.map.gz | 250 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1795-v30.xml emd-1795-v30.xml emd-1795.xml emd-1795.xml | 9.1 KB 9.1 KB | Display Display |  EMDB header EMDB header |
| Images |  tp901-baseplate-bppU.png tp901-baseplate-bppU.png | 34.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1795 http://ftp.pdbj.org/pub/emdb/structures/EMD-1795 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1795 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1795 | HTTPS FTP |
-Validation report
| Summary document |  emd_1795_validation.pdf.gz emd_1795_validation.pdf.gz | 184 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_1795_full_validation.pdf.gz emd_1795_full_validation.pdf.gz | 183.1 KB | Display | |
| Data in XML |  emd_1795_validation.xml.gz emd_1795_validation.xml.gz | 5.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1795 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1795 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1795 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1795 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_1795.map.gz / Format: CCP4 / Size: 11.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1795.map.gz / Format: CCP4 / Size: 11.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The three-dimensional representation of the baseplate of TP901-1 bppU mutant | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.64 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : TP901-1 bacteriophage bppU mutant
| Entire | Name: TP901-1 bacteriophage bppU mutant |
|---|---|
| Components |
|
-Supramolecule #1000: TP901-1 bacteriophage bppU mutant
| Supramolecule | Name: TP901-1 bacteriophage bppU mutant / type: sample / ID: 1000 / Oligomeric state: The baseplate is hexameric / Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 1 MDa / Theoretical: 1 MDa |
-Macromolecule #1: TP901-1 bppU mutant
| Macromolecule | Name: TP901-1 bppU mutant / type: protein_or_peptide / ID: 1 / Name.synonym: TP901-1 bppU mutant / Oligomeric state: Hexameric / Recombinant expression: Yes |
|---|---|
| Source (natural) | Organism:  Lactococcus phage TP901-1 (virus) Lactococcus phage TP901-1 (virus) |
| Molecular weight | Experimental: 1 MDa / Theoretical: 1 MDa |
| Recombinant expression | Organism:  |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 Details: 100 mM sodium chloride, 10 mM magnesium sulphate, 50 mM Tris pH 7.5, 0.01% (w/v) gelatin |
|---|---|
| Staining | Type: NEGATIVE Details: Grids with adsorbed protein floated on 2% uranyl acetate for one minute. |
| Grid | Details: Copper grid |
| Vitrification | Cryogen name: NONE / Instrument: OTHER |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Image recording | Category: CCD / Film or detector model: GENERIC TVIPS (4k x 4k) / Digitization - Sampling interval: 4.64 µm / Average electron dose: 10 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source: OTHER |
| Electron optics | Calibrated magnification: 38000 / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.2 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 38000 |
| Sample stage | Specimen holder: Room temperature / Specimen holder model: OTHER |
- Image processing
Image processing
| Details | The reconstruction was refined using projection matching |
|---|---|
| CTF correction | Details: CCD Images |
| Final reconstruction | Applied symmetry - Point group: C6 (6 fold cyclic) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 28.0 Å / Resolution method: OTHER / Software - Name: IMAGIC Details: The reconstruction was refined using projection matching Number images used: 2000 |
| Final angle assignment | Details: IMAGIC, beta 90 degrees |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















