[English] 日本語
 Yorodumi
Yorodumi- EMDB-17313: In situ cryoEM structure of the Prototype Foamy Virus capsid, pen... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In situ cryoEM structure of the Prototype Foamy Virus capsid, pentamer localised reconstruction | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | capsid / Gag / foamy virus / pentamer / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cytoskeleton / microtubule-dependent intracellular transport of viral material towards nucleus / viral release from host cell / host cell / viral nucleocapsid / host cell cytoplasm / symbiont entry into host cell / host cell nucleus Similarity search - Function | |||||||||
| Biological species |  Eastern chimpanzee simian foamy virus Eastern chimpanzee simian foamy virus | |||||||||
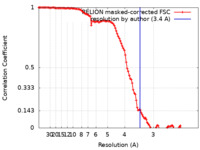
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Calcraft T / Nans A / Rosenthal PB | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2024 Journal: Cell / Year: 2024Title: Integrated cryoEM structure of a spumaretrovirus reveals cross-kingdom evolutionary relationships and the molecular basis for assembly and virus entry. Authors: Thomas Calcraft / Nicole Stanke-Scheffler / Andrea Nans / Dirk Lindemann / Ian A Taylor / Peter B Rosenthal /   Abstract: Foamy viruses (FVs) are an ancient lineage of retroviruses, with an evolutionary history spanning over 450 million years. Vector systems based on Prototype Foamy Virus (PFV) are promising candidates ...Foamy viruses (FVs) are an ancient lineage of retroviruses, with an evolutionary history spanning over 450 million years. Vector systems based on Prototype Foamy Virus (PFV) are promising candidates for gene and oncolytic therapies. Structural studies of PFV contribute to the understanding of the mechanisms of FV replication, cell entry and infection, and retroviral evolution. Here we combine cryoEM and cryoET to determine high-resolution in situ structures of the PFV icosahedral capsid (CA) and envelope glycoprotein (Env), including its type III transmembrane anchor and membrane-proximal external region (MPER), and show how they are organized in an integrated structure of assembled PFV particles. The atomic models reveal an ancient retroviral capsid architecture and an unexpected relationship between Env and other class 1 fusion proteins of the Mononegavirales. Our results represent the de novo structure determination of an assembled retrovirus particle. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17313.map.gz emd_17313.map.gz | 116.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17313-v30.xml emd-17313-v30.xml emd-17313.xml emd-17313.xml | 18.8 KB 18.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17313_fsc.xml emd_17313_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_17313.png emd_17313.png | 87.9 KB | ||
| Masks |  emd_17313_msk_1.map emd_17313_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-17313.cif.gz emd-17313.cif.gz | 6.2 KB | ||
| Others |  emd_17313_half_map_1.map.gz emd_17313_half_map_1.map.gz emd_17313_half_map_2.map.gz emd_17313_half_map_2.map.gz | 96.8 MB 96.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17313 http://ftp.pdbj.org/pub/emdb/structures/EMD-17313 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17313 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17313 | HTTPS FTP |
-Validation report
| Summary document |  emd_17313_validation.pdf.gz emd_17313_validation.pdf.gz | 904.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_17313_full_validation.pdf.gz emd_17313_full_validation.pdf.gz | 904 KB | Display | |
| Data in XML |  emd_17313_validation.xml.gz emd_17313_validation.xml.gz | 18.8 KB | Display | |
| Data in CIF |  emd_17313_validation.cif.gz emd_17313_validation.cif.gz | 24.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17313 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17313 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17313 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17313 | HTTPS FTP |
-Related structure data
| Related structure data |  8ozlMC  8ozhC  8ozjC  8ozkC  8ozmC  8oznC  8ozpC  8ozqC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_17313.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17313.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
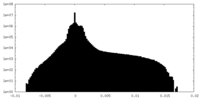
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_17313_msk_1.map emd_17313_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_17313_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_17313_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Eastern chimpanzee simian foamy virus
| Entire | Name:  Eastern chimpanzee simian foamy virus Eastern chimpanzee simian foamy virus |
|---|---|
| Components |
|
-Supramolecule #1: Eastern chimpanzee simian foamy virus
| Supramolecule | Name: Eastern chimpanzee simian foamy virus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 2170195 / Sci species name: Eastern chimpanzee simian foamy virus / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: Yes / Virus empty: No |
|---|
-Macromolecule #1: Gag polyprotein
| Macromolecule | Name: Gag polyprotein / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Eastern chimpanzee simian foamy virus Eastern chimpanzee simian foamy virus |
| Molecular weight | Theoretical: 70.693414 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MASGSNVEEY ELDVEALVVI LRDRNIPRNP LHGEVIGLRL TEGWWGQIER FQMVRLILQN DDNEPLQRPR YEVIQRAVNP HTMFMISGP LAELQLAFQD LDLPEGPLRF GPLANGHYVQ GDPYSSSYRP VTMAETAQMT RDELEDVLNT QSEIEIQMIN L LELYEVET ...String: MASGSNVEEY ELDVEALVVI LRDRNIPRNP LHGEVIGLRL TEGWWGQIER FQMVRLILQN DDNEPLQRPR YEVIQRAVNP HTMFMISGP LAELQLAFQD LDLPEGPLRF GPLANGHYVQ GDPYSSSYRP VTMAETAQMT RDELEDVLNT QSEIEIQMIN L LELYEVET RALRRQLAER SSTGQGGISP GAPRSRPPVS SFSGLPSLPS IPGIHPRAPS PPRATSTPGN IPWSLGDDNP PS SSFPGPS QPRVSFHPGN PFVEEEGHRP RSQSRERRRE ILPAPVPSAP PMIQYIPVPP PPPIGTVIPI QHIRSVTGEP PRN PREIPI WLGRNAPAID GVFPVTTPDL RCRIINAILG GNIGLSLTPG DCLTWDSAVA TLFIRTHGTF PMHQLGNVIK GIVD QEGVA TAYTLGMMLS GQNYQLVSGI IRGYLPGQAV VTALQQRLDQ EIDDQTRAET FIQHLNAVYE ILGLNARGQS IRASV TPQP RPSRGRGRGQ NTSRPSQGPA NSGRGRQRPA SGQSNRGSST QNQNQDNLNQ GGYNLRPRTY QPQRYGGGRG RRWNDN TNN QESRPSDQGS QTPRPNQAGS GVRGNQSQTP RPAAGRGGRG NHNRNQRSSG AGDSRAVNTV TQSATSSTDE SSSAVTA AS GGDQRD UniProtKB: Gag polyprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-8ozl: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)