[English] 日本語
 Yorodumi
Yorodumi- EMDB-16007: Cryo-EM structure of the Arabidopsis thaliana I+III2 supercomplex... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Arabidopsis thaliana I+III2 supercomplex (CIII membrane domain) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Plant / Mitochondria / Complex / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationvacuole / respiratory chain complex III / quinol-cytochrome-c reductase / quinol-cytochrome-c reductase activity / plastid / mitochondrial electron transport, ubiquinol to cytochrome c / 2 iron, 2 sulfur cluster binding / mitochondrial membrane / electron transfer activity / oxidoreductase activity ...vacuole / respiratory chain complex III / quinol-cytochrome-c reductase / quinol-cytochrome-c reductase activity / plastid / mitochondrial electron transport, ubiquinol to cytochrome c / 2 iron, 2 sulfur cluster binding / mitochondrial membrane / electron transfer activity / oxidoreductase activity / mitochondrial inner membrane / heme binding / mitochondrion / metal ion binding / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
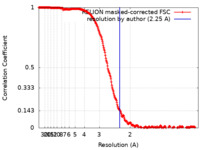
| Method | single particle reconstruction / cryo EM / Resolution: 2.25 Å | |||||||||
 Authors Authors | Klusch N / Kuehlbrandt W | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Plants / Year: 2023 Journal: Nat Plants / Year: 2023Title: Cryo-EM structure of the respiratory I + III supercomplex from Arabidopsis thaliana at 2 Å resolution. Authors: Niklas Klusch / Maximilian Dreimann / Jennifer Senkler / Nils Rugen / Werner Kühlbrandt / Hans-Peter Braun /  Abstract: Protein complexes of the mitochondrial respiratory chain assemble into respiratory supercomplexes. Here we present the high-resolution electron cryo-microscopy structure of the Arabidopsis ...Protein complexes of the mitochondrial respiratory chain assemble into respiratory supercomplexes. Here we present the high-resolution electron cryo-microscopy structure of the Arabidopsis respiratory supercomplex consisting of complex I and a complex III dimer, with a total of 68 protein subunits and numerous bound cofactors. A complex I-ferredoxin, subunit B14.7 and P9, a newly defined subunit of plant complex I, mediate supercomplex formation. The component complexes stabilize one another, enabling new detailed insights into their structure. We describe (1) an interrupted aqueous passage for proton translocation in the membrane arm of complex I; (2) a new coenzyme A within the carbonic anhydrase module of plant complex I defining a second catalytic centre; and (3) the water structure at the proton exit pathway of complex III with a co-purified ubiquinone in the Q site. We propose that the main role of the plant supercomplex is to stabilize its components in the membrane. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16007.map.gz emd_16007.map.gz | 64.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16007-v30.xml emd-16007-v30.xml emd-16007.xml emd-16007.xml | 27.7 KB 27.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16007_fsc.xml emd_16007_fsc.xml | 26.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_16007.png emd_16007.png | 173.4 KB | ||
| Filedesc metadata |  emd-16007.cif.gz emd-16007.cif.gz | 7.5 KB | ||
| Others |  emd_16007_additional_1.map.gz emd_16007_additional_1.map.gz emd_16007_half_map_1.map.gz emd_16007_half_map_1.map.gz emd_16007_half_map_2.map.gz emd_16007_half_map_2.map.gz | 91.1 MB 1 GB 1 GB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16007 http://ftp.pdbj.org/pub/emdb/structures/EMD-16007 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16007 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16007 | HTTPS FTP |
-Related structure data
| Related structure data |  8belMC  8bedC  8beeC  8befC  8behC  8bepC  8bpxC  8bq5C  8bq6C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_16007.map.gz / Format: CCP4 / Size: 68.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16007.map.gz / Format: CCP4 / Size: 68.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.573 Å | ||||||||||||||||||||||||||||||||||||
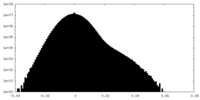
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_16007_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
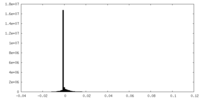
| Density Histograms |
-Half map: #2
| File | emd_16007_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
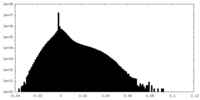
| Density Histograms |
-Half map: #1
| File | emd_16007_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
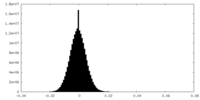
| Density Histograms |
- Sample components
Sample components
+Entire : Mitochondrial Arabidopsis thaliana I+III2 supercomplex (CIII memb...
+Supramolecule #1: Mitochondrial Arabidopsis thaliana I+III2 supercomplex (CIII memb...
+Macromolecule #1: Cytochrome b
+Macromolecule #2: Cytochrome b-c1 complex subunit Rieske-1, mitochondrial
+Macromolecule #3: Cytochrome c1 2, heme protein, mitochondrial
+Macromolecule #4: Cytochrome b-c1 complex subunit 8-1, mitochondrial
+Macromolecule #5: Cytochrome b-c1 complex subunit 6-1, mitochondrial
+Macromolecule #6: Cytochrome b-c1 complex subunit 9, mitochondrial
+Macromolecule #7: Cytochrome b-c1 complex subunit 10, mitochondrial
+Macromolecule #8: PROTOPORPHYRIN IX CONTAINING FE
+Macromolecule #9: 2,3-DIMETHOXY-5-METHYL-6-(3,11,15,19-TETRAMETHYL-EICOSA-2,6,10,14...
+Macromolecule #10: 1,2-DIACYL-GLYCEROL-3-SN-PHOSPHATE
+Macromolecule #11: (1S)-2-{[{[(2R)-2,3-DIHYDROXYPROPYL]OXY}(HYDROXY)PHOSPHORYL]OXY}-...
+Macromolecule #12: CARDIOLIPIN
+Macromolecule #13: (7S)-4-HYDROXY-N,N,N-TRIMETHYL-9-OXO-7-[(PALMITOYLOXY)METHYL]-3,5...
+Macromolecule #14: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #15: PHOSPHATIDYLETHANOLAMINE
+Macromolecule #16: UBIQUINONE-7
+Macromolecule #17: 2-{[(4-O-alpha-D-glucopyranosyl-alpha-D-glucopyranosyl)oxy]methyl...
+Macromolecule #18: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.18 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 400 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: CONTINUOUS / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: GRAPHENE / Support film - #1 - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 70 % / Chamber temperature: 283.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 215000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Software | Name:  Coot (ver. 0.9.5) Coot (ver. 0.9.5) |
|---|---|
| Refinement | Space: REAL |
| Output model |  PDB-8bel: |
 Movie
Movie Controller
Controller




















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)