+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1571 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | VP7 recoated rotavirus DLP | |||||||||
 Map data Map data | One quadrant of the 7RP capsid. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | rotavirus / capsid / VP7 / VP6 / VP2 | |||||||||
| Function / homology |  Function and homology information Function and homology informationviral intermediate capsid / host cell endoplasmic reticulum lumen / T=2 icosahedral viral capsid / T=13 icosahedral viral capsid / viral inner capsid / viral outer capsid / viral nucleocapsid / receptor-mediated virion attachment to host cell / host cell surface receptor binding / fusion of virus membrane with host plasma membrane ...viral intermediate capsid / host cell endoplasmic reticulum lumen / T=2 icosahedral viral capsid / T=13 icosahedral viral capsid / viral inner capsid / viral outer capsid / viral nucleocapsid / receptor-mediated virion attachment to host cell / host cell surface receptor binding / fusion of virus membrane with host plasma membrane / viral envelope / structural molecule activity / RNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Rotavirus Rotavirus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.5 Å | |||||||||
 Authors Authors | Chen JZ / Settembre EC / Aoki ST / Zhang X / Bellamy AR / Dormitzer PR / Harrison SC / Grigorieff N | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2009 Journal: Proc Natl Acad Sci U S A / Year: 2009Title: Molecular interactions in rotavirus assembly and uncoating seen by high-resolution cryo-EM. Authors: James Z Chen / Ethan C Settembre / Scott T Aoki / Xing Zhang / A Richard Bellamy / Philip R Dormitzer / Stephen C Harrison / Nikolaus Grigorieff /  Abstract: Rotaviruses, major causes of childhood gastroenteritis, are nonenveloped, icosahedral particles with double-strand RNA genomes. By the use of electron cryomicroscopy and single-particle ...Rotaviruses, major causes of childhood gastroenteritis, are nonenveloped, icosahedral particles with double-strand RNA genomes. By the use of electron cryomicroscopy and single-particle reconstruction, we have visualized a rotavirus particle comprising the inner capsid coated with the trimeric outer-layer protein, VP7, at a resolution (4 A) comparable with that of X-ray crystallography. We have traced the VP7 polypeptide chain, including parts not seen in its X-ray crystal structure. The 3 well-ordered, 30-residue, N-terminal "arms" of each VP7 trimer grip the underlying trimer of VP6, an inner-capsid protein. Structural differences between free and particle-bound VP7 and between free and VP7-coated inner capsids may regulate mRNA transcription and release. The Ca(2+)-stabilized VP7 intratrimer contact region, which presents important neutralizing epitopes, is unaltered upon capsid binding. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1571.map.gz emd_1571.map.gz | 440.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1571-v30.xml emd-1571-v30.xml emd-1571.xml emd-1571.xml | 10.1 KB 10.1 KB | Display Display |  EMDB header EMDB header |
| Images |  7RP_icon.tif 7RP_icon.tif | 732.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1571 http://ftp.pdbj.org/pub/emdb/structures/EMD-1571 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1571 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1571 | HTTPS FTP |
-Validation report
| Summary document |  emd_1571_validation.pdf.gz emd_1571_validation.pdf.gz | 345.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_1571_full_validation.pdf.gz emd_1571_full_validation.pdf.gz | 344.8 KB | Display | |
| Data in XML |  emd_1571_validation.xml.gz emd_1571_validation.xml.gz | 10.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1571 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1571 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1571 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1571 | HTTPS FTP |
-Related structure data
| Related structure data |  3gztMC  3gzuMC  1609C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1571.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1571.map.gz / Format: CCP4 / Size: 1.9 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | One quadrant of the 7RP capsid. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.233 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
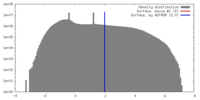
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : VP7 recoated rotavirus DLP
| Entire | Name: VP7 recoated rotavirus DLP |
|---|---|
| Components |
|
-Supramolecule #1000: VP7 recoated rotavirus DLP
| Supramolecule | Name: VP7 recoated rotavirus DLP / type: sample / ID: 1000 / Oligomeric state: Icosahedral virus capsid / Number unique components: 1 |
|---|---|
| Molecular weight | Experimental: 60 MDa / Theoretical: 60 MDa / Method: Sum of all components |
-Supramolecule #1: Rotavirus
| Supramolecule | Name: Rotavirus / type: virus / ID: 1 / Name.synonym: Rotavirus / Details: Inner-layer VP2, mid-layer VP6, outer-layer VP7 / NCBI-ID: 10912 / Sci species name: Rotavirus / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: SEROTYPE / Virus enveloped: No / Virus empty: No / Syn species name: Rotavirus |
|---|---|
| Host (natural) | Organism:  |
| Molecular weight | Experimental: 60 MDa / Theoretical: 60 MDa |
| Virus shell | Shell ID: 1 / Name: VP2 VP6 VP7 / Diameter: 1000 Å / T number (triangulation number): 13 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.0 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 20mM Tris-HCl, 50mM NaCl, 2mM CaCl2 |
| Grid | Details: C-falt grids |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 90 % / Chamber temperature: 90 K / Instrument: HOMEMADE PLUNGER / Details: Vitrification instrument: Manual plunger / Method: Blot for 3 seconds before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Temperature | Min: 90 K / Max: 90 K / Average: 90 K |
| Date | Dec 1, 2007 |
| Image recording | Category: CCD / Film or detector model: KODAK SO-163 FILM / Digitization - Sampling interval: 7 µm / Number real images: 148 / Average electron dose: 25 e/Å2 / Od range: 1.2 / Bits/pixel: 12 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 58168 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.0 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.2 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder: Eucentric / Specimen holder model: GATAN LIQUID NITROGEN |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | The particles were selected using SIGNATURE, the density map was reconstructed and refined using FREALIGN. |
|---|---|
| CTF correction | Details: Individual particle |
| Final reconstruction | Applied symmetry - Point group: I (icosahedral) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 5.5 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: FREALIGN / Number images used: 3780 |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)