[English] 日本語
 Yorodumi
Yorodumi- EMDB-15683: Human leptin in complex with the human LEP-R ectodomain fused to ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human leptin in complex with the human LEP-R ectodomain fused to a C-terminal trimeric isoleucine GCN4 zipper (open 3:3 model). | |||||||||
 Map data Map data | Sharpened cryo-EM map following non-uniform refinement in cryoSPARC | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | leptin / LEP-R / obesity / metabolism / energy balance / CYTOKINE | |||||||||
| Function / homology |  Function and homology information Function and homology informationmulticellular organism development / leptin receptor activity / regulation of lipoprotein lipid oxidation / cellular response to L-ascorbic acid / positive regulation of fat cell apoptotic process / negative regulation of glutamine transport / negative regulation of appetite by leptin-mediated signaling pathway / negative regulation of glucagon secretion / regulation of endothelial cell proliferation / regulation of transport ...multicellular organism development / leptin receptor activity / regulation of lipoprotein lipid oxidation / cellular response to L-ascorbic acid / positive regulation of fat cell apoptotic process / negative regulation of glutamine transport / negative regulation of appetite by leptin-mediated signaling pathway / negative regulation of glucagon secretion / regulation of endothelial cell proliferation / regulation of transport / leptin receptor binding / regulation of natural killer cell mediated cytotoxicity / regulation of bone remodeling / positive regulation of luteinizing hormone secretion / regulation of natural killer cell proliferation / regulation of natural killer cell activation / bone growth / glycerol biosynthetic process / regulation of steroid biosynthetic process / elastin metabolic process / leptin-mediated signaling pathway / positive regulation of follicle-stimulating hormone secretion / regulation of intestinal cholesterol absorption / positive regulation of monoatomic ion transport / regulation of feeding behavior / regulation of brown fat cell differentiation / positive regulation of hepatic stellate cell activation / positive regulation of peroxisome proliferator activated receptor signaling pathway / regulation of nitric-oxide synthase activity / adult feeding behavior / sexual reproduction / activation of protein kinase C activity / bone mineralization involved in bone maturation / response to leptin / negative regulation of cartilage development / negative regulation of D-glucose import across plasma membrane / negative regulation of appetite / ovulation from ovarian follicle / positive regulation of developmental growth / energy reserve metabolic process / leukocyte tethering or rolling / cellular response to leptin stimulus / prostaglandin secretion / cardiac muscle hypertrophy / bile acid metabolic process / Signaling by Leptin / positive regulation of p38MAPK cascade / hormone metabolic process / cytokine receptor activity / cell surface receptor signaling pathway via STAT / intestinal absorption / eating behavior / insulin secretion / regulation of gluconeogenesis / aorta development / negative regulation of vasoconstriction / response to vitamin E / glycogen metabolic process / peptide hormone receptor binding / cytokine binding / fatty acid beta-oxidation / regulation of cytokine production involved in inflammatory response / central nervous system neuron development / response to dietary excess / peptide hormone binding / T cell differentiation / negative regulation of lipid storage / Synthesis, secretion, and deacylation of Ghrelin / regulation of angiogenesis / negative regulation of gluconeogenesis / positive regulation of TOR signaling / cell surface receptor signaling pathway via JAK-STAT / adipose tissue development / positive regulation of insulin receptor signaling pathway / phagocytosis / glial cell proliferation / transport across blood-brain barrier / cholesterol metabolic process / energy homeostasis / cellular response to retinoic acid / positive regulation of interleukin-12 production / regulation of insulin secretion / positive regulation of T cell proliferation / negative regulation of autophagy / placenta development / response to activity / gluconeogenesis / determination of adult lifespan / positive regulation of interleukin-8 production / positive regulation of receptor signaling pathway via JAK-STAT / lipid metabolic process / female pregnancy / circadian rhythm / hormone activity / positive regulation of protein import into nucleus / Transcriptional regulation of white adipocyte differentiation / response to insulin / positive regulation of interleukin-6 production / regulation of blood pressure / cellular response to insulin stimulus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
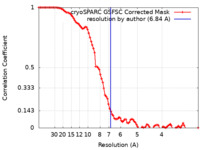
| Method | single particle reconstruction / cryo EM / Resolution: 6.84 Å | |||||||||
 Authors Authors | Verstraete K / Savvides SN / Verschueren KG / Tsirigotaki A | |||||||||
| Funding support |  Belgium, 1 items Belgium, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Mechanism of receptor assembly via the pleiotropic adipokine Leptin. Authors: Alexandra Tsirigotaki / Ann Dansercoer / Koen H G Verschueren / Iva Marković / Christoph Pollmann / Maximillian Hafer / Jan Felix / Catherine Birck / Wouter Van Putte / Dominiek Catteeuw / ...Authors: Alexandra Tsirigotaki / Ann Dansercoer / Koen H G Verschueren / Iva Marković / Christoph Pollmann / Maximillian Hafer / Jan Felix / Catherine Birck / Wouter Van Putte / Dominiek Catteeuw / Jan Tavernier / J Fernando Bazan / Jacob Piehler / Savvas N Savvides / Kenneth Verstraete /     Abstract: The adipokine Leptin activates its receptor LEP-R in the hypothalamus to regulate body weight and exerts additional pleiotropic functions in immunity, fertility and cancer. However, the structure and ...The adipokine Leptin activates its receptor LEP-R in the hypothalamus to regulate body weight and exerts additional pleiotropic functions in immunity, fertility and cancer. However, the structure and mechanism of Leptin-mediated LEP-R assemblies has remained unclear. Intriguingly, the signaling-competent isoform of LEP-R is only lowly abundant amid several inactive short LEP-R isoforms contributing to a mechanistic conundrum. Here we show by X-ray crystallography and cryo-EM that, in contrast to long-standing paradigms, Leptin induces type I cytokine receptor assemblies featuring 3:3 stoichiometry and demonstrate such Leptin-induced trimerization of LEP-R on living cells via single-molecule microscopy. In mediating these assemblies, Leptin undergoes drastic restructuring that activates its site III for binding to the Ig domain of an adjacent LEP-R. These interactions are abolished by mutations linked to obesity. Collectively, our study provides the structural and mechanistic framework for how evolutionarily conserved Leptin:LEP-R assemblies with 3:3 stoichiometry can engage distinct LEP-R isoforms to achieve signaling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15683.map.gz emd_15683.map.gz | 78.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15683-v30.xml emd-15683-v30.xml emd-15683.xml emd-15683.xml | 25.7 KB 25.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15683_fsc.xml emd_15683_fsc.xml | 9.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_15683.png emd_15683.png | 34.1 KB | ||
| Masks |  emd_15683_msk_1.map emd_15683_msk_1.map | 83.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15683.cif.gz emd-15683.cif.gz | 7.9 KB | ||
| Others |  emd_15683_additional_1.map.gz emd_15683_additional_1.map.gz emd_15683_half_map_1.map.gz emd_15683_half_map_1.map.gz emd_15683_half_map_2.map.gz emd_15683_half_map_2.map.gz | 41.7 MB 77.8 MB 77.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15683 http://ftp.pdbj.org/pub/emdb/structures/EMD-15683 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15683 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15683 | HTTPS FTP |
-Related structure data
| Related structure data |  8avoMC  7z3pC  7z3qC  7z3rC  8av2C  8avbC  8avcC  8avdC  8aveC  8avfC  8b7qC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15683.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15683.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened cryo-EM map following non-uniform refinement in cryoSPARC | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.52 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15683_msk_1.map emd_15683_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
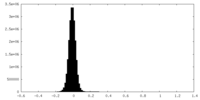
| Density Histograms |
-Additional map: Non-sharpened cryo-EM map following non-uniform refinement in cryoSPARC...
| File | emd_15683_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Non-sharpened cryo-EM map following non-uniform refinement in cryoSPARC | ||||||||||||
| Projections & Slices |
| ||||||||||||
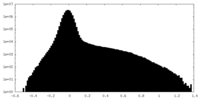
| Density Histograms |
-Half map: Half map B
| File | emd_15683_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
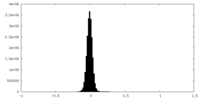
| Density Histograms |
-Half map: Half map A
| File | emd_15683_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
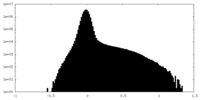
| Density Histograms |
- Sample components
Sample components
-Entire : Human leptin in complex with the human LEP-R ectodomain C-termina...
| Entire | Name: Human leptin in complex with the human LEP-R ectodomain C-terminally fused to a trimeric GCN4 isoleucine zipper tag |
|---|---|
| Components |
|
-Supramolecule #1: Human leptin in complex with the human LEP-R ectodomain C-termina...
| Supramolecule | Name: Human leptin in complex with the human LEP-R ectodomain C-terminally fused to a trimeric GCN4 isoleucine zipper tag type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Human leptin carrying an N-terminal His-tag and the human LEP-R ectodomain C-terminally fused to a trimeric GCN4 isoleucine zipper tag (without His-tag) were co-expressed in HEK293 FreeStyle ...Details: Human leptin carrying an N-terminal His-tag and the human LEP-R ectodomain C-terminally fused to a trimeric GCN4 isoleucine zipper tag (without His-tag) were co-expressed in HEK293 FreeStyle cells in the presence of kifunensine. The resulting complex was purified from the conditioned medium by IMAC and SEC. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 350 KDa |
-Macromolecule #1: Leptin
| Macromolecule | Name: Leptin / type: protein_or_peptide / ID: 1 Details: N-terminally His-tagged human leptin was co-expressed with the human LEP-R ectodomain fused a trimeric GCN4 isoleucine zipper tag in HEK293 FreeStyle cells. Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 18.605061 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AHHHHHHPGG PGSENLYFQG GSTGGVPIQK VQDDTKTLIK TIVTRINDIS HTQSVSSKQK VTGLDFIPGL HPILTLSKMD QTLAVYQQI LTSMPSRNVI QISNDLENLR DLLHVLAFSK SCHLPWASGL ETLDSLGGVL EASGYSTEVV ALSRLQGSLQ D MLWQLDLS PGC UniProtKB: Leptin |
-Macromolecule #2: Leptin receptor
| Macromolecule | Name: Leptin receptor / type: protein_or_peptide / ID: 2 Details: Human leptin carrying an N-terminal His-tag and the human LEP-R ectodomain C-terminally fused to a trimeric GCN4 isoleucine zipper tag (without His-tag) were co-expressed in HEK293 FreeStyle ...Details: Human leptin carrying an N-terminal His-tag and the human LEP-R ectodomain C-terminally fused to a trimeric GCN4 isoleucine zipper tag (without His-tag) were co-expressed in HEK293 FreeStyle cells in the presence of kifunensine. The resulting complex was purified from the conditioned medium by IMAC and SEC. Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 98.822062 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: FNLSYPITPW RFKLSCMPPN STYDYFLLPA GLSKNTSNSN GHYETAVEPK FNSSGTHFSN LSKTTFHCCF RSEQDRNCSL CADNIEGKT FVSTVNSLVF QQIDANWNIQ CWLKGDLKLF ICYVESLFKN LFRNYNYKVH LLYVLPEVLE DSPLVPQKGS F QMVHCNCS ...String: FNLSYPITPW RFKLSCMPPN STYDYFLLPA GLSKNTSNSN GHYETAVEPK FNSSGTHFSN LSKTTFHCCF RSEQDRNCSL CADNIEGKT FVSTVNSLVF QQIDANWNIQ CWLKGDLKLF ICYVESLFKN LFRNYNYKVH LLYVLPEVLE DSPLVPQKGS F QMVHCNCS VHECCECLVP VPTAKLNDTL LMCLKITSGG VIFQSPLMSV QPINMVKPDP PLGLHMEITD DGNLKISWSS PP LVPFPLQ YQVKYSENST TVIREADKIV SATSLLVDSI LPGSSYEVQV RGKRLDGPGI WSDWSTPRVF TTQDVIYFPP KIL TSVGSN VSFHCIYKKE NKIVPSKEIV WWMNLAEKIP QSQYDVVSDH VSKVTFFNLN ETKPRGKFTY DAVYCCNEHE CHHR YAELY VIDVNINISC ETDGYLTKMT CRWSTSTIQS LAESTLQLRY HRSSLYCSDI PSIHPISEPK DCYLQSDGFY ECIFQ PIFL LSGYTMWIRI NHSLGSLDSP PTCVLPDSVV KPLPPSSVKA EITINIGLLK ISWEKPVFPE NNLQFQIRYG LSGKEV QWK MYEVYDAKSK SVSLPVPDLC AVYAVQVRCK RLDGLGYWSN WSNPAYTVVM DIKVPMRGPE FWRIINGDTM KKEKNVT LL WKPLMKNDSL CSVQRYVINH HTSCNGTWSE DVGNHTKFTF LWTEQAHTVT VLAINSIGAS VANFNLTFSW PMSKVNIV Q SLSAYPLNSS CVIVSWILSP SDYKLMYFII EWKNLNEDGE IKWLRISSSV KKYYIHDHFI PIEKYQFSLY PIFMEGVGK PKIINSFTQD DIEKHQSDST GGSGGSGGSG GSGGSRMKQI EDKIEEILSK IYHIENEIAR IKKLIGER UniProtKB: Leptin receptor |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.3 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: 20 mM HEPES, 150 mM NaCl, pH 7.4 | |||||||||
| Grid | Model: Quantifoil R0.6/1 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 1 sec. / Pretreatment - Atmosphere: AIR Details: 4 microliter of hLeptin:hLEP-RECD-tGCN4 complex at 0.3 mg.mL-1 was applied to a glow-discharged Quantifoil R 0.6/1 300 mesh golden grid coated with graphene (PUXANO), blotted for 1 s (blot ...Details: 4 microliter of hLeptin:hLEP-RECD-tGCN4 complex at 0.3 mg.mL-1 was applied to a glow-discharged Quantifoil R 0.6/1 300 mesh golden grid coated with graphene (PUXANO), blotted for 1 s (blot force = 1) under 100% humidity at 295K and plunged into liquid ethane using an FEI Vitribot Mark IV | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV | |||||||||
| Details | This sample was monodisperse. |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 13755 / Average electron dose: 62.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: OTHER / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.8 µm |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Details | Atomic models for the 2:2 and 3:3 hLeptin:hLEP-R complexes were created based on the Alphafold2 predictions for hLEP-R and hLeptin, and the crystal structures for the mouse 3:3 Leptin:LEP-RIgCRH2 complex (pdb 7z3r) and the human Leptin:LEP-RCRH2 complex (pdb 7z3q) and fitted in the cryo-EM maps via Chimera. Atomic models were further refined via real space refinement in Phenix using rigid body refinement and coordinate refinement with reference restraints to the starting model. | ||||||
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT | ||||||
| Output model |  PDB-8avo: |
 Movie
Movie Controller
Controller




















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)