[English] 日本語
 Yorodumi
Yorodumi- EMDB-15677: Cryo-EM structure for mouse leptin in complex with the mouse LEP-... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure for mouse leptin in complex with the mouse LEP-R ectodomain (1:2 mLEP:mLEPR model) | |||||||||
 Map data Map data | Sharpened cryo-EM map following non-uniform refinement | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | leptin / LEP-R / obesity / metabolism / energy balance / CYTOKINE | |||||||||
| Function / homology |  Function and homology information Function and homology informationSynthesis, secretion, and deacylation of Ghrelin / leptin receptor activity / regulation of lipoprotein lipid oxidation / cellular response to L-ascorbic acid / positive regulation of fat cell apoptotic process / negative regulation of glutamine transport / negative regulation of appetite by leptin-mediated signaling pathway / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / negative regulation of glucagon secretion / regulation of endothelial cell proliferation ...Synthesis, secretion, and deacylation of Ghrelin / leptin receptor activity / regulation of lipoprotein lipid oxidation / cellular response to L-ascorbic acid / positive regulation of fat cell apoptotic process / negative regulation of glutamine transport / negative regulation of appetite by leptin-mediated signaling pathway / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / negative regulation of glucagon secretion / regulation of endothelial cell proliferation / regulation of transport / leptin receptor binding / regulation of natural killer cell mediated cytotoxicity / regulation of bone remodeling / regulation of natural killer cell proliferation / positive regulation of luteinizing hormone secretion / regulation of natural killer cell activation / bone growth / glycerol biosynthetic process / regulation of steroid biosynthetic process / elastin metabolic process / leptin-mediated signaling pathway / positive regulation of follicle-stimulating hormone secretion / regulation of intestinal cholesterol absorption / positive regulation of monoatomic ion transport / regulation of feeding behavior / regulation of brown fat cell differentiation / positive regulation of hepatic stellate cell activation / positive regulation of peroxisome proliferator activated receptor signaling pathway / regulation of nitric-oxide synthase activity / sexual reproduction / adult feeding behavior / activation of protein kinase C activity / bone mineralization involved in bone maturation / response to leptin / regulation of lipid biosynthetic process / negative regulation of cartilage development / negative regulation of D-glucose import across plasma membrane / negative regulation of appetite / ovulation from ovarian follicle / positive regulation of developmental growth / energy reserve metabolic process / leukocyte tethering or rolling / cellular response to leptin stimulus / prostaglandin secretion / cardiac muscle hypertrophy / bile acid metabolic process / positive regulation of p38MAPK cascade / regulation of protein localization to nucleus / hormone metabolic process / cell surface receptor signaling pathway via STAT / regulation of metabolic process / regulation of fat cell differentiation / intestinal absorption / insulin secretion / eating behavior / regulation of gluconeogenesis / aorta development / negative regulation of vasoconstriction / response to vitamin E / glycogen metabolic process / peptide hormone receptor binding / fatty acid beta-oxidation / regulation of cytokine production involved in inflammatory response / central nervous system neuron development / response to dietary excess / peptide hormone binding / T cell differentiation / negative regulation of lipid storage / regulation of angiogenesis / negative regulation of gluconeogenesis / positive regulation of TOR signaling / cell surface receptor signaling pathway via JAK-STAT / adipose tissue development / positive regulation of insulin receptor signaling pathway / phagocytosis / glial cell proliferation / cholesterol metabolic process / energy homeostasis / cellular response to retinoic acid / positive regulation of interleukin-12 production / regulation of insulin secretion / positive regulation of T cell proliferation / negative regulation of autophagy / placenta development / response to activity / gluconeogenesis / determination of adult lifespan / positive regulation of interleukin-8 production / positive regulation of receptor signaling pathway via JAK-STAT / lipid metabolic process / female pregnancy / circadian rhythm / hormone activity / positive regulation of protein import into nucleus / response to insulin / positive regulation of interleukin-6 production / regulation of blood pressure / cellular response to insulin stimulus / positive regulation of reactive oxygen species metabolic process Similarity search - Function | |||||||||
| Biological species |  | |||||||||
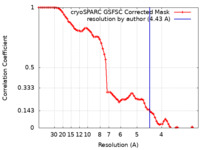
| Method | single particle reconstruction / cryo EM / Resolution: 4.43 Å | |||||||||
 Authors Authors | Verstraete K / Savvides SN / Verschueren KG / Tsirigotaki A | |||||||||
| Funding support |  Belgium, 1 items Belgium, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Mechanism of receptor assembly via the pleiotropic adipokine Leptin. Authors: Alexandra Tsirigotaki / Ann Dansercoer / Koen H G Verschueren / Iva Marković / Christoph Pollmann / Maximillian Hafer / Jan Felix / Catherine Birck / Wouter Van Putte / Dominiek Catteeuw / ...Authors: Alexandra Tsirigotaki / Ann Dansercoer / Koen H G Verschueren / Iva Marković / Christoph Pollmann / Maximillian Hafer / Jan Felix / Catherine Birck / Wouter Van Putte / Dominiek Catteeuw / Jan Tavernier / J Fernando Bazan / Jacob Piehler / Savvas N Savvides / Kenneth Verstraete /     Abstract: The adipokine Leptin activates its receptor LEP-R in the hypothalamus to regulate body weight and exerts additional pleiotropic functions in immunity, fertility and cancer. However, the structure and ...The adipokine Leptin activates its receptor LEP-R in the hypothalamus to regulate body weight and exerts additional pleiotropic functions in immunity, fertility and cancer. However, the structure and mechanism of Leptin-mediated LEP-R assemblies has remained unclear. Intriguingly, the signaling-competent isoform of LEP-R is only lowly abundant amid several inactive short LEP-R isoforms contributing to a mechanistic conundrum. Here we show by X-ray crystallography and cryo-EM that, in contrast to long-standing paradigms, Leptin induces type I cytokine receptor assemblies featuring 3:3 stoichiometry and demonstrate such Leptin-induced trimerization of LEP-R on living cells via single-molecule microscopy. In mediating these assemblies, Leptin undergoes drastic restructuring that activates its site III for binding to the Ig domain of an adjacent LEP-R. These interactions are abolished by mutations linked to obesity. Collectively, our study provides the structural and mechanistic framework for how evolutionarily conserved Leptin:LEP-R assemblies with 3:3 stoichiometry can engage distinct LEP-R isoforms to achieve signaling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15677.map.gz emd_15677.map.gz | 38.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15677-v30.xml emd-15677-v30.xml emd-15677.xml emd-15677.xml | 24.1 KB 24.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15677_fsc.xml emd_15677_fsc.xml | 7.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_15677.png emd_15677.png | 101.8 KB | ||
| Masks |  emd_15677_msk_1.map emd_15677_msk_1.map | 40.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15677.cif.gz emd-15677.cif.gz | 7.5 KB | ||
| Others |  emd_15677_additional_1.map.gz emd_15677_additional_1.map.gz emd_15677_half_map_1.map.gz emd_15677_half_map_1.map.gz emd_15677_half_map_2.map.gz emd_15677_half_map_2.map.gz | 20.4 MB 37.7 MB 37.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15677 http://ftp.pdbj.org/pub/emdb/structures/EMD-15677 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15677 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15677 | HTTPS FTP |
-Related structure data
| Related structure data |  8avbMC  7z3pC  7z3qC  7z3rC  8av2C  8avcC  8avdC  8aveC  8avfC  8avoC  8b7qC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15677.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15677.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened cryo-EM map following non-uniform refinement | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.51 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15677_msk_1.map emd_15677_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Non-sharpened cryo-EM map following non-uniform refinement
| File | emd_15677_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Non-sharpened cryo-EM map following non-uniform refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map A
| File | emd_15677_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map B
| File | emd_15677_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex between mouse leptin and the mouse LEP-R ectodomain.
| Entire | Name: Complex between mouse leptin and the mouse LEP-R ectodomain. |
|---|---|
| Components |
|
-Supramolecule #1: Complex between mouse leptin and the mouse LEP-R ectodomain.
| Supramolecule | Name: Complex between mouse leptin and the mouse LEP-R ectodomain. type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: The mouse leptin:LEP-R complex was isolated from the excess of mouse leptin via size-exclusion chromatography. The elution peak corresponding to the leptin:LEP-R was concentrated to 5 mg/mL, ...Details: The mouse leptin:LEP-R complex was isolated from the excess of mouse leptin via size-exclusion chromatography. The elution peak corresponding to the leptin:LEP-R was concentrated to 5 mg/mL, aliquoted and flash frozen into liquid nitrogen. Just before plunge freezing the sample was diluted to 0.2 mg/mL. |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 230 KDa |
-Macromolecule #1: Leptin
| Macromolecule | Name: Leptin / type: protein_or_peptide / ID: 1 Details: Mouse leptin was expressed with an N-terminal His-tag. Before complex formation with the mouse LEP-R ecotodomain, the His-tag was removed with TEV protease Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 16.434676 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GGSTGGVPIQ KVQDDTKTLI KTIVTRINDI SHTQSVSAKQ RVTGLDFIPG LHPILSLSKM DQTLAVYQQV LTSLPSQNVL QIANDLENL RDLLHLLAFS KSCSLPQTSG LQKPESLDGV LEASLYSTEV VALSRLQGSL QDILQQLDVS PEC UniProtKB: Leptin |
-Macromolecule #2: Leptin receptor
| Macromolecule | Name: Leptin receptor / type: protein_or_peptide / ID: 2 Details: The N-terminally His-tagged LEP-R ecotomain was secreted from HEK293 FreeStyle cells. The N-terminal His-tag was not removed before complex formation with refolded mouse leptin. Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 94.081344 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AHHHHHHPGG PGSDELDLNL AYPISPWKFK LFCGPPNTTD DSFLSPAGAP NNASALKGAS EAIVEAKFNS SGIYVPELSK TVFHCCFGN EQGQNCSALT DNTEGKTLAS VVKASVFRQL GVNWDIECWM KGDLTLFICH MEPLPKNPFK NYDSKVHLLY D LPEVIDDS ...String: AHHHHHHPGG PGSDELDLNL AYPISPWKFK LFCGPPNTTD DSFLSPAGAP NNASALKGAS EAIVEAKFNS SGIYVPELSK TVFHCCFGN EQGQNCSALT DNTEGKTLAS VVKASVFRQL GVNWDIECWM KGDLTLFICH MEPLPKNPFK NYDSKVHLLY D LPEVIDDS PLPPLKDSFQ TVQCNCSLRG CECHVPVPRA KLNYALLMYL EITSAGVSFQ SPLMSLQPML VVKPDPPLGL HM EVTDDGN LKISWDSQTM APFPLQYQVK YLENSTIVRE AAEIVSATSL LVDSVLPGSS YEVQVRSKRL DGSGVWSDWS SPQ VFTTQD VVYFPPKILT SVGSNASFHC IYKNENQIIS SKQIVWWRNL AEKIPEIQYS IVSDRVSKVT FSNLKATRPR GKFT YDAVY CCNEQACHHR YAELYVIDVN INISCETDGY LTKMTCRWSP STIQSLVGST VQLRYHRRSL YCPDSPSIHP TSEPK NCVL QRDGFYECVF QPIFLLSGYT MWIRINHSLG SLDSPPTCVL PDSVVKPLPP SNVKAEITVN TGLLKVSWEK PVFPEN NLQ FQIRYGLSGK EIQWKTHEVF DAKSKSASLL VSDLCAVYVV QVRCRRLDGL GYWSNWSSPA YTLVMDVKVP MRGPEFW RK MDGDVTKKER NVTLLWKPLT KNDSLCSVRR YVVKHRTAHN GTWSEDVGNR TNLTFLWTEP AHTVTVLAVN SLGASLVN F NLTFSWPMSK VSAVESLSAY PLSSSCVILS WTLSPDDYSL LYLVIEWKIL NEDDGMKWLR IPSNVKKFYI HDNFIPIEK YQFSLYPVFM EGVGKPKIIN GFTKDAIDKQ QNDAG UniProtKB: Leptin receptor |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: 20 mM Hepes, 150 mM NaCl, pH 7.4 | |||||||||
| Grid | Model: Quantifoil R0.6/1 / Material: GOLD / Mesh: 300 / Support film - Material: GRAPHENE / Support film - topology: CONTINUOUS / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 1 sec. / Pretreatment - Atmosphere: AIR | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV | |||||||||
| Details | The mouse leptin:LEP-R complex was isolated from the excess of mouse leptin via size-exclusion chromatography. The elution peak corresponding to the leptin:LEP-R was concentrated to 5 mg/mL, aliquoted and flash frozen into liquid nitrogen. Just before plunge freezing the sample was diluted to 0.2 mg/mL. |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 7100 / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: OTHER / Nominal defocus max: 3.0 µm / Nominal defocus min: 0.8 µm |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | An atomic model for a 1:2 mLeptin:LEP-RIgCRH2FnIII complex was created based on the AlphaFold prediction for mLEP-RECD and the determined mLeptin:mLEP-RIgCRH2 and mLEP-RFnIII module crystal structures and fitted in the cryo-EM map via Chimera followed by real space refinement in Phenix using rigid body refinement and coordinate refinement with reference restraints to the starting model and hydrogen-bonding restraints across the site II and site III mLeptin:mLEP-R interface regions. |
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
| Output model |  PDB-8avb: |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)