[English] 日本語
 Yorodumi
Yorodumi- EMDB-15464: In situ subtomogram average of floating microtubule inner protein... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In situ subtomogram average of floating microtubule inner protein 13 (fMIP13) | |||||||||
 Map data Map data | In situ subtomogram average of floating microtubule inner protein 13 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | microtubule-associated protein / STRUCTURAL PROTEIN | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
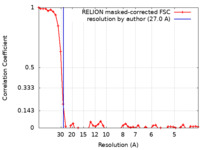
| Method | subtomogram averaging / cryo EM / Resolution: 27.0 Å | |||||||||
 Authors Authors | Chakraborty S / Mahamid J / Martinez-Sanchez A / Baumeister W | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2025 Journal: Proc Natl Acad Sci U S A / Year: 2025Title: Cryo-ET suggests tubulin chaperones form a subset of microtubule lumenal particles with a role in maintaining neuronal microtubules. Authors: Saikat Chakraborty / Antonio Martinez-Sanchez / Florian Beck / Mauricio Toro-Nahuelpan / In-Young Hwang / Kyung-Min Noh / Wolfgang Baumeister / Julia Mahamid /  Abstract: The functional architecture of the long-lived neuronal microtubule (MT) cytoskeleton is maintained by various MT-associated proteins (MAPs), most of which are known to bind to the MT outer surface. ...The functional architecture of the long-lived neuronal microtubule (MT) cytoskeleton is maintained by various MT-associated proteins (MAPs), most of which are known to bind to the MT outer surface. However, electron microscopy (EM) has long ago revealed the presence of particles inside the lumens of neuronal MTs, of yet unknown identity and function. Here, we use cryogenic electron tomography (cryo-ET) to analyze the three-dimensional (3D) organization and structures of MT lumenal particles in primary hippocampal neurons, human induced pluripotent stem cell-derived neurons, and pluripotent and differentiated P19 cells. We obtain in situ density maps of several lumenal particles from the respective cells and detect common structural features underscoring their potential overarching functions. Mass spectrometry-based proteomics combined with structural modeling suggest that a subset of lumenal particles could be tubulin-binding cofactors (TBCs) bound to tubulin monomers. A different subset of smaller particles, which remains unidentified, exhibits densities that bridge across the MT protofilaments. We show that increased lumenal particle concentration within MTs is concomitant with neuronal differentiation and correlates with higher MT curvatures. Enrichment of lumenal particles around MT lattice defects and at freshly polymerized MT open-ends suggests a MT protective role. Together with the identified structural resemblance of a subset of particles to TBCs, these results hint at a role in local tubulin proteostasis for the maintenance of long-lived neuronal MTs. #1:  Journal: Biorxiv / Year: 2024 Journal: Biorxiv / Year: 2024Title: Cryo-electron tomography suggests tubulin chaperones form a subset of microtubule lumenal particles with a role in maintaining neuronal microtubules Authors: Chakraborty S / Martinez-Sanchez A / Beck F / Toro-Nahuelpan M / Hwang IY / Noh KM / Baumeister W / Mahamid J | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15464.map.gz emd_15464.map.gz | 3.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15464-v30.xml emd-15464-v30.xml emd-15464.xml emd-15464.xml | 17 KB 17 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15464_fsc.xml emd_15464_fsc.xml | 4.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_15464.png emd_15464.png | 10.2 KB | ||
| Filedesc metadata |  emd-15464.cif.gz emd-15464.cif.gz | 4.4 KB | ||
| Others |  emd_15464_half_map_1.map.gz emd_15464_half_map_1.map.gz emd_15464_half_map_2.map.gz emd_15464_half_map_2.map.gz | 6 MB 6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15464 http://ftp.pdbj.org/pub/emdb/structures/EMD-15464 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15464 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15464 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15464.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15464.map.gz / Format: CCP4 / Size: 8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | In situ subtomogram average of floating microtubule inner protein 13 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.129 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: In situ subtomogram average of floating microtubule inner protein 13
| File | emd_15464_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | In situ subtomogram average of floating microtubule inner protein 13 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: In situ subtomogram average of floating microtubule inner protein 13
| File | emd_15464_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | In situ subtomogram average of floating microtubule inner protein 13 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : fMIP13 obtained from human induced pluripotent stem cells derived...
| Entire | Name: fMIP13 obtained from human induced pluripotent stem cells derived neuron. |
|---|---|
| Components |
|
-Supramolecule #1: fMIP13 obtained from human induced pluripotent stem cells derived...
| Supramolecule | Name: fMIP13 obtained from human induced pluripotent stem cells derived neuron. type: cell / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Grid | Model: Quantifoil / Material: GOLD / Mesh: 200 / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 70 % / Chamber temperature: 310 K / Instrument: LEICA EM GP |
| Details | Frozen hydrated human induced pluripotent stem cells derived neurons. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 77.0 K |
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 2.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 2.5 µm / Nominal magnification: 64000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)