+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13927 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | RNA-bound human SKI complex | ||||||||||||||||||
 Map data Map data | postprocessed_map_hSKI_RNA-bound | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | multiprotein complex / RNA helicase / DExH-box helicase / ATPase / RNA binding / RNA BINDING PROTEIN | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationSki complex / mRNA decay by 3' to 5' exoribonuclease / Cdc73/Paf1 complex / nuclear-transcribed mRNA catabolic process, 3'-5' exonucleolytic nonsense-mediated decay / negative regulation of myeloid cell differentiation / 3'-5' RNA helicase activity / Association of TriC/CCT with target proteins during biosynthesis / nuclear-transcribed mRNA catabolic process / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex ...Ski complex / mRNA decay by 3' to 5' exoribonuclease / Cdc73/Paf1 complex / nuclear-transcribed mRNA catabolic process, 3'-5' exonucleolytic nonsense-mediated decay / negative regulation of myeloid cell differentiation / 3'-5' RNA helicase activity / Association of TriC/CCT with target proteins during biosynthesis / nuclear-transcribed mRNA catabolic process / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / RNA Polymerase II Pre-transcription Events / rescue of stalled cytosolic ribosome / transcription elongation by RNA polymerase II / euchromatin / Wnt signaling pathway / E3 ubiquitin ligases ubiquitinate target proteins / RNA helicase activity / RNA helicase / ATP hydrolysis activity / RNA binding / nucleoplasm / ATP binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | ||||||||||||||||||
 Authors Authors | Koegel A / Keidel A | ||||||||||||||||||
| Funding support |  Germany, 5 items Germany, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2022 Journal: Mol Cell / Year: 2022Title: The human SKI complex regulates channeling of ribosome-bound RNA to the exosome via an intrinsic gatekeeping mechanism. Authors: Alexander Kögel / Achim Keidel / Fabien Bonneau / Ingmar B Schäfer / Elena Conti /  Abstract: The superkiller (SKI) complex is the cytoplasmic co-factor and regulator of the RNA-degrading exosome. In human cells, the SKI complex functions mainly in co-translational surveillance-decay ...The superkiller (SKI) complex is the cytoplasmic co-factor and regulator of the RNA-degrading exosome. In human cells, the SKI complex functions mainly in co-translational surveillance-decay pathways, and its malfunction is linked to a severe congenital disorder, the trichohepatoenteric syndrome. To obtain insights into the molecular mechanisms regulating the human SKI (hSKI) complex, we structurally characterized several of its functional states in the context of 80S ribosomes and substrate RNA. In a prehydrolytic ATP form, the hSKI complex exhibits a closed conformation with an inherent gating system that effectively traps the 80S-bound RNA into the hSKI2 helicase subunit. When active, hSKI switches to an open conformation in which the gating is released and the RNA 3' end exits the helicase. The emerging picture is that the gatekeeping mechanism and architectural remodeling of hSKI underpin a regulated RNA channeling system that is mechanistically conserved among the cytoplasmic and nuclear helicase-exosome complexes. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13927.map.gz emd_13927.map.gz | 31.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13927-v30.xml emd-13927-v30.xml emd-13927.xml emd-13927.xml | 21.9 KB 21.9 KB | Display Display |  EMDB header EMDB header |
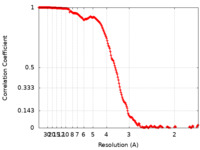
| FSC (resolution estimation) |  emd_13927_fsc.xml emd_13927_fsc.xml | 18.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_13927.png emd_13927.png | 75.4 KB | ||
| Masks |  emd_13927_msk_1.map emd_13927_msk_1.map | 512 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13927.cif.gz emd-13927.cif.gz | 7.9 KB | ||
| Others |  emd_13927_half_map_1.map.gz emd_13927_half_map_1.map.gz emd_13927_half_map_2.map.gz emd_13927_half_map_2.map.gz | 410.8 MB 410.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13927 http://ftp.pdbj.org/pub/emdb/structures/EMD-13927 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13927 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13927 | HTTPS FTP |
-Related structure data
| Related structure data |  7qdyMC  7qdrC  7qdsC  7qdzC  7qe0C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13927.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13927.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | postprocessed_map_hSKI_RNA-bound | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8512 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
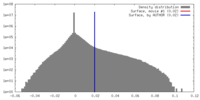
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13927_msk_1.map emd_13927_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
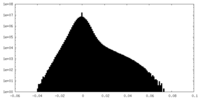
| Density Histograms |
-Half map: half map 1
| File | emd_13927_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_map_1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_13927_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half_map_2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : H.s. SKI complex bound to RNA
| Entire | Name: H.s. SKI complex bound to RNA |
|---|---|
| Components |
|
-Supramolecule #1: H.s. SKI complex bound to RNA
| Supramolecule | Name: H.s. SKI complex bound to RNA / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 380 KDa |
-Macromolecule #1: Helicase SKI2W
| Macromolecule | Name: Helicase SKI2W / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 137.913688 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MMETERLVLP PPDPLDLPLR AVELGCTGHW ELLNLPGAPE SSLPHGLPPC APDLQQEAEQ LFLSSPAWLP LHGVEHSARK WQRKTDPWS LLAVLGAPVP SDLQAQRHPT TGQILGYKEV LLENTNLSAT TSLSLRRPPG PASQSLWGNP TQYPFWPGGM D EPTITDLN ...String: MMETERLVLP PPDPLDLPLR AVELGCTGHW ELLNLPGAPE SSLPHGLPPC APDLQQEAEQ LFLSSPAWLP LHGVEHSARK WQRKTDPWS LLAVLGAPVP SDLQAQRHPT TGQILGYKEV LLENTNLSAT TSLSLRRPPG PASQSLWGNP TQYPFWPGGM D EPTITDLN TREEAEEEID FEKDLLTIPP GFKKGMDFAP KDCPTPAPGL LSLSCMLEPL DLGGGDEDEN EAVGQPGGPR GD TVSASPC SAPLARASSL EDLVLKEAST AVSTPEAPEP PSQEQWAIPV DATSPVGDFY RLIPQPAFQW AFEPDVFQKQ AIL HLERHD SVFVAAHTSA GKTVVAEYAI ALAQKHMTRT IYTSPIKALS NQKFRDFRNT FGDVGLLTGD VQLHPEASCL IMTT EILRS MLYSGSDVIR DLEWVIFDEV HYINDVERGV VWEEVLIMLP DHVSIILLSA TVPNALEFAD WIGRLKRRQI YVIST VTRP VPLEHYLFTG NSSKTQGELF LLLDSRGAFH TKGYYAAVEA KKERMSKHAQ TFGAKQPTHQ GGPAQDRGVY LSLLAS LRT RAQLPVVVFT FSRGRCDEQA SGLTSLDLTT SSEKSEIHLF LQRCLARLRG SDRQLPQVLH MSELLNRGLG VHHSGIL PI LKEIVEMLFS RGLVKVLFAT ETFAMGVNMP ARTVVFDSMR KHDGSTFRDL LPGEYVQMAG RAGRRGLDPT GTVILLCK G RVPEMADLHR MMMGKPSQLQ SQFRLTYTMI LNLLRVDALR VEDMMKRSFS EFPSRKDSKA HEQALAELTK RLGALEEPD MTGQLVDLPE YYSWGEELTE TQHMIQRRIM ESVNGLKSLS AGRVVVVKNQ EHHNALGVIL QVSSNSTSRV FTTLVLCDKP LSQDPQDRG PATAEVPYPD DLVGFKLFLP EGPCDHTVVK LQPGDMAAIT TKVLRVNGEK ILEDFSKRQQ PKFKKDPPLA A VTTAVQEL LRLAQAHPAG PPTLDPVNDL QLKDMSVVEG GLRARKLEEL IQGAQCVHSP RFPAQYLKLR ERMQIQKEME RL RFLLSDQ SLLLLPEYHQ RVEVLRTLGY VDEAGTVKLA GRVACAMSSH ELLLTELMFD NALSTLRPEE IAALLSGLVC QSP GDAGDQ LPNTLKQGIE RVRAVAKRIG EVQVACGLNQ TVEEFVGELN FGLVEVVYEW ARGMPFSELA GLSGTPEGLV VRCI QRLAE MCRSLRGAAR LVGEPVLGAK METAATLLRR DIVFAASLYT Q UniProtKB: Superkiller complex protein 2 |
-Macromolecule #2: Tetratricopeptide repeat protein 37
| Macromolecule | Name: Tetratricopeptide repeat protein 37 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 178.651641 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKHHHHHHHH HHSAGLEVLF QGPDSMSSKE VKTALKSARD AIRNKEYKEA LKHCKTVLKQ EKNNYNAWVF IGVAAAELEQ PDQAQSAYK KAAELEPDQL LAWQGLANLY EKYNHINAKD DLPGVYQKLL DLYESVDKQK WCDVCKKLVD LYYQEKKHLE V ARTWHKLI ...String: MKHHHHHHHH HHSAGLEVLF QGPDSMSSKE VKTALKSARD AIRNKEYKEA LKHCKTVLKQ EKNNYNAWVF IGVAAAELEQ PDQAQSAYK KAAELEPDQL LAWQGLANLY EKYNHINAKD DLPGVYQKLL DLYESVDKQK WCDVCKKLVD LYYQEKKHLE V ARTWHKLI KTRQEQGAEN EELHQLWRKL TQFLAESTED QNNETQQLLF TAFENALGLS DKIPSEDHQV LYRHFIQSLS KF PHESARL KKACEGMINI YPTVQYPLEV LCLHLIESGN LTDEGQQYCC RLVEMDSKSG PGLIGLGIKA LQDKKYEDAV RNL TEGLKE SPVCTSGWYH LAEAQVKMHR PKEAVLSCSQ ALKIVDNLGA SGNSLYQRNL CLHLKAEALI KLSDYDSSEE AIRT LDQIS DADNIPGLLV LKSLAYRNKG SFDEAAKIME DLLSSYPDLA EVHALEALIH FTKKDYLQAE KCFQRALEKD TEVAE YHYQ LGLTYWFMGE ETRKDKTKAL THFLKAARLD TYMGKVFCYL GHYYRDVVGD KNRARGCYRK AFELDDTDAE SGAAAV DLS VELEDMEMAL AILTTVTQKA SAGTAKWAWL RRGLYYLKAG QHSQAVADLQ AALRADPKDF NCWESLGEAY LSRGGYT TA LKSFTKASEL NPESIYSVFK VAAIQQILGK YKEAVAQYQM IIKKKEDYVP ALKGLGECHL MMAKAALVDY LDGKAVDY I EKALEYFTCA LQHRADVSCL WKLAGDACTC LYAVAPSKVN VHVLGVLLGQ KEGKQVLKKN ELLHLGGRCY GRALKLMST SNTWCDLGIN YYRQAQHLAE TGSNMNDLKE LLEKSLHCLK KAVRLDSNNH LYWNALGVVA CYSGIGNYAL AQHCFIKSIQ SEQINAVAW TNLGVLYLTN ENIEQAHEAF KMAQSLDPSY LMCWIGQALI AEAVGSYDTM DLFRHTTELN MHTEGALGYA Y WVCTTLQD KSNRETELYQ YNILQMNAIP AAQVILNKYV ERIQNYAPAF TMLGYLNEHL QLKKEAANAY QRAILLLQTA ED QDTYNVA IRNYGRLLCS TGEYDKAIQA FKSTPLEVLE DIIGFALALF MKGLYKESSK AYERALSIVE SEQDKAHILT ALA ITEYKQ GKTDVAKTLL FKCSILKEPT TESLQALCAL GLAMQDATLS KAALNELLKH IKHKDSNYQR CLLTSAIYAL QGRS VAVQK QISKAVHSNP GDPALWSLLS RVVAQYAQRN AKGGVVAGNV AHILDSNHGK KALLYTAVNQ LAMGSSSAED EKNTA LKTI QKAALLSPGD PAIWAGLMAA CHADDKLALV NNTQPKRIDL YLALLSAVSA SIKDEKFFEN YNQSLEKWSL SQAVTG LID TGRISEAETL CTKNLKSNPD QPAVILLLRQ VQCKPLLESQ KPLPDAVLEE LQKTVMSNST SVPAWQWLAH VYQSQGM MR AAEMCYRKSL QLASQRGSWS GKLSSLLRLA LLALKVCMAN ISNDHWPSLV QEATTEALKL CFCPLAVLLQ ALLQFKRK M GARETRRLLE RVVYQPGYPK SIASTARWYL LRHLYAKDDY ELIDVLVNNA KTHGDTRALE LNQRLSSQ UniProtKB: Superkiller complex protein 3 |
-Macromolecule #3: WD repeat-containing protein 61
| Macromolecule | Name: WD repeat-containing protein 61 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 33.617465 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTNQYGILFK QEQAHDDAIW SVAWGTNKKE NSETVVTGSL DDLVKVWKWR DERLDLQWSL EGHQLGVVSV DISHTLPIAA SSSLDAHIR LWDLENGKQI KSIDAGPVDA WTLAFSPDSQ YLATGTHVGK VNIFGVESGK KEYSLDTRGK FILSIAYSPD G KYLASGAI ...String: MTNQYGILFK QEQAHDDAIW SVAWGTNKKE NSETVVTGSL DDLVKVWKWR DERLDLQWSL EGHQLGVVSV DISHTLPIAA SSSLDAHIR LWDLENGKQI KSIDAGPVDA WTLAFSPDSQ YLATGTHVGK VNIFGVESGK KEYSLDTRGK FILSIAYSPD G KYLASGAI DGIINIFDIA TGKLLHTLEG HAMPIRSLTF SPDSQLLVTA SDDGYIKIYD VQHANLAGTL SGHASWVLNV AF CPDDTHF VSSSSDKSVK VWDVGTRTCV HTFFDHQDQV WGVKYNGNGS KIVSVGDDQE IHIYDCPI UniProtKB: Superkiller complex protein 8 |
-Macromolecule #4: RNA (5'-R(P*UP*UP*UP*UP*UP*U)-3')
| Macromolecule | Name: RNA (5'-R(P*UP*UP*UP*UP*UP*U)-3') / type: rna / ID: 4 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.609189 KDa |
| Sequence | String: UUUUUUUUUU UUUUUUUUUU UUUUU |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 200 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average exposure time: 3.0 sec. / Average electron dose: 68.31 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: BACKBONE TRACE / Target criteria: CORRELATION COEFFICIENT |
|---|---|
| Output model |  PDB-7qdy: |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)