[English] 日本語
 Yorodumi
Yorodumi- EMDB-13345: Cryo-EM structure of BMV-derived VLP expressed in E. coli (eVLP) -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13345 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of BMV-derived VLP expressed in E. coli (eVLP) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | BMV / brome mosaic virus / capsid proteins / VIRUS LIKE PARTICLE | |||||||||
| Function / homology | Bromovirus coat protein / Bromovirus coat protein / viral capsid / structural molecule activity / Coat protein Function and homology information Function and homology information | |||||||||
| Biological species |   Brome mosaic virus Brome mosaic virus | |||||||||
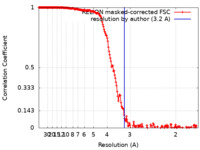
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | |||||||||
 Authors Authors | Ruszkowski M / Strugala A | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nanoscale / Year: 2022 Journal: Nanoscale / Year: 2022Title: Cryo-EM reconstructions of BMV-derived virus-like particles reveal assembly defects in the icosahedral lattice structure. Authors: Milosz Ruszkowski / Aleksander Strugala / Paulina Indyka / Guillaume Tresset / Marek Figlerowicz / Anna Urbanowicz /   Abstract: The increasing interest in virus-like particles (VLPs) has been reflected by the growing number of studies on their assembly and application. However, the formation of complete VLPs is a complex ...The increasing interest in virus-like particles (VLPs) has been reflected by the growing number of studies on their assembly and application. However, the formation of complete VLPs is a complex phenomenon, making it difficult to rationally design VLPs with desired features . In this paper, we describe VLPs assembled from the recombinant capsid protein of brome mosaic virus (BMV). The analysis of VLPs was performed by Cryo-EM reconstructions and allowed us to visualize a few classes of VLPs, giving insight into the VLP self-assembly process. Apart from the mature icosahedral VLP practically identical with native virions, we describe putative VLP intermediates displaying non-icosahedral arrangements of capsomers, proposed to occur before the final disorder-order transition stage of icosahedral VLP assembly. Some of the described VLP classes show a lack of protein shell continuity, possibly resulting from too strong interaction with the cargo (in this case tRNA) with the capsid protein. We believe that our results are a useful prerequisite for the rational design of VLPs in the future and lead the way to the effective production of modified VLPs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13345.map.gz emd_13345.map.gz | 474.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13345-v30.xml emd-13345-v30.xml emd-13345.xml emd-13345.xml | 11.8 KB 11.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13345_fsc.xml emd_13345_fsc.xml | 18.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_13345.png emd_13345.png | 255.3 KB | ||
| Filedesc metadata |  emd-13345.cif.gz emd-13345.cif.gz | 5.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13345 http://ftp.pdbj.org/pub/emdb/structures/EMD-13345 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13345 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13345 | HTTPS FTP |
-Validation report
| Summary document |  emd_13345_validation.pdf.gz emd_13345_validation.pdf.gz | 802.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_13345_full_validation.pdf.gz emd_13345_full_validation.pdf.gz | 802 KB | Display | |
| Data in XML |  emd_13345_validation.xml.gz emd_13345_validation.xml.gz | 15.9 KB | Display | |
| Data in CIF |  emd_13345_validation.cif.gz emd_13345_validation.cif.gz | 22.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13345 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13345 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13345 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-13345 | HTTPS FTP |
-Related structure data
| Related structure data |  7pe2MC  7pe1C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_13345.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13345.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.86 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Brome mosaic virus
| Entire | Name:   Brome mosaic virus Brome mosaic virus |
|---|---|
| Components |
|
-Supramolecule #1: Brome mosaic virus
| Supramolecule | Name: Brome mosaic virus / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1 / NCBI-ID: 12302 / Sci species name: Brome mosaic virus / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: OTHER / Virus enveloped: No / Virus empty: Yes |
|---|
-Macromolecule #1: Coat protein
| Macromolecule | Name: Coat protein / type: protein_or_peptide / ID: 1 / Number of copies: 180 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Brome mosaic virus Brome mosaic virus |
| Molecular weight | Theoretical: 20.568693 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SNIMSTSGTG KMTRAQRRAA ARRNRRTAGV QPVIVEPIAA GQGKAIKAIA GYSISKWEAS SDAITAKATN AMSITLPHEL SSEKNKELK VGRVLLWLGL LPSVAGRIKA CVAEKQAQAE AAFQVALAVA DSSKEVVAAM YTDAFRGATL GDLLNLQIYL Y ASEAVPAK ...String: SNIMSTSGTG KMTRAQRRAA ARRNRRTAGV QPVIVEPIAA GQGKAIKAIA GYSISKWEAS SDAITAKATN AMSITLPHEL SSEKNKELK VGRVLLWLGL LPSVAGRIKA CVAEKQAQAE AAFQVALAVA DSSKEVVAAM YTDAFRGATL GDLLNLQIYL Y ASEAVPAK AVVVHLEVEH VRPTFDDFFT PVYK UniProtKB: Coat protein |
-Macromolecule #2: water
| Macromolecule | Name: water / type: ligand / ID: 2 / Number of copies: 180 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL |
|---|---|
| Buffer | pH: 4.8 Details: 25 mM NaOAc pH 4.8, 5 mM MgCl2, 25 mM NaCl, 10 mM KCl |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 12569 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 3.0 µm / Calibrated defocus min: 0.9 µm / Calibrated magnification: 105000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)