[English] 日本語
 Yorodumi
Yorodumi- EMDB-13230: Cryo-EM structure of homomeric LRRC8A Volume-Regulated Anion Chan... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-13230 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of homomeric LRRC8A Volume-Regulated Anion Channel in complex with synthetic nanobody Sb5 | |||||||||
 Map data Map data | full-length complex at 3.8 A | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | LRRC8 family / Volume-Regulated Anion Channel / leucine-rich repeat / sybody / cryo-EM / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationMiscellaneous transport and binding events / pre-B cell differentiation / aspartate transmembrane transport / volume-sensitive anion channel activity / cyclic-GMP-AMP transmembrane transporter activity / cyclic-GMP-AMP transmembrane import across plasma membrane / taurine transmembrane transport / monoatomic anion transmembrane transport / protein hexamerization / monoatomic anion transport ...Miscellaneous transport and binding events / pre-B cell differentiation / aspartate transmembrane transport / volume-sensitive anion channel activity / cyclic-GMP-AMP transmembrane transporter activity / cyclic-GMP-AMP transmembrane import across plasma membrane / taurine transmembrane transport / monoatomic anion transmembrane transport / protein hexamerization / monoatomic anion transport / cell volume homeostasis / response to osmotic stress / intracellular glucose homeostasis / monoatomic ion channel complex / positive regulation of myoblast differentiation / chloride transmembrane transport / positive regulation of insulin secretion / spermatogenesis / lysosomal membrane / cell surface / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
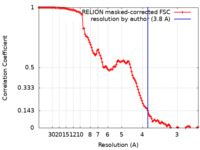
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Deneka D / Rutz S | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2021 Journal: Nat Commun / Year: 2021Title: Allosteric modulation of LRRC8 channels by targeting their cytoplasmic domains. Authors: Dawid Deneka / Sonja Rutz / Cedric A J Hutter / Markus A Seeger / Marta Sawicka / Raimund Dutzler /  Abstract: Members of the LRRC8 family form heteromeric assemblies, which function as volume-regulated anion channels. These modular proteins consist of a transmembrane pore and cytoplasmic leucine-rich repeat ...Members of the LRRC8 family form heteromeric assemblies, which function as volume-regulated anion channels. These modular proteins consist of a transmembrane pore and cytoplasmic leucine-rich repeat (LRR) domains. Despite their known molecular architecture, the mechanism of activation and the role of the LRR domains in this process has remained elusive. Here we address this question by generating synthetic nanobodies, termed sybodies, which target the LRR domain of the obligatory subunit LRRC8A. We use these binders to investigate their interaction with homomeric LRRC8A channels by cryo-electron microscopy and the consequent effect on channel activation by electrophysiology. The five identified sybodies either inhibit or enhance activity by binding to distinct epitopes of the LRR domain, thereby altering channel conformations. In combination, our work provides a set of specific modulators of LRRC8 proteins and reveals the role of their cytoplasmic domains as regulators of channel activity by allosteric mechanisms. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_13230.map.gz emd_13230.map.gz | 10.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-13230-v30.xml emd-13230-v30.xml emd-13230.xml emd-13230.xml | 19.3 KB 19.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_13230_fsc.xml emd_13230_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_13230.png emd_13230.png | 66.7 KB | ||
| Masks |  emd_13230_msk_1.map emd_13230_msk_1.map | 144.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-13230.cif.gz emd-13230.cif.gz | 6.4 KB | ||
| Others |  emd_13230_half_map_1.map.gz emd_13230_half_map_1.map.gz emd_13230_half_map_2.map.gz emd_13230_half_map_2.map.gz | 8.2 MB 8.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-13230 http://ftp.pdbj.org/pub/emdb/structures/EMD-13230 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13230 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-13230 | HTTPS FTP |
-Related structure data
| Related structure data |  7p6kMC  7p5vC  7p5wC  7p5yC  7p60C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_13230.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_13230.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | full-length complex at 3.8 A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.302 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_13230_msk_1.map emd_13230_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_13230_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_13230_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Homomeric LRRC8A Volume-Regulated Anion Channel in complex with s...
| Entire | Name: Homomeric LRRC8A Volume-Regulated Anion Channel in complex with synthetic nanobody Sb5 |
|---|---|
| Components |
|
-Supramolecule #1: Homomeric LRRC8A Volume-Regulated Anion Channel in complex with s...
| Supramolecule | Name: Homomeric LRRC8A Volume-Regulated Anion Channel in complex with synthetic nanobody Sb5 type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Macromolecule #1: Volume-regulated anion channel subunit LRRC8A
| Macromolecule | Name: Volume-regulated anion channel subunit LRRC8A / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 94.239383 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MIPVTELRYF ADTQPAYRIL KPWWDVFTDY ISIVMLMIAV FGGTLQVTQD KMICLPCKWV TKDSCNDSFR GWAASSPEPT YPNSTVLPT PDTGPTGIKY DLDRHQYNYV DAVCYENRLH WFAKYFPYLV LLHTLIFLAC SNFWFKFPRT SSKLEHFVSI L LKCFDSPW ...String: MIPVTELRYF ADTQPAYRIL KPWWDVFTDY ISIVMLMIAV FGGTLQVTQD KMICLPCKWV TKDSCNDSFR GWAASSPEPT YPNSTVLPT PDTGPTGIKY DLDRHQYNYV DAVCYENRLH WFAKYFPYLV LLHTLIFLAC SNFWFKFPRT SSKLEHFVSI L LKCFDSPW TTRALSETVV EESDPKPAFS KMNGSMDKKS STVSEDVEAT VPMLQRTKSR IEQGIVDRSE TGVLDKKEGE QA KALFEKV KKFRTHVEEG DIVYRLYMRQ TIIKVIKFAL IICYTVYYVH NIKFDVDCTV DIESLTGYRT YRCAHPLATL FKI LASFYI SLVIFYGLIC MYTLWWMLRR SLKKYSFESI REESSYSDIP DVKNDFAFML HLIDQYDPLY SKRFAVFLSE VSEN KLRQL NLNNEWTLDK LRQRLTKNAQ DKLELHLFML SGIPDTVFDL VELEVLKLEL IPDVTIPPSI AQLTGLKELW LYHTA AKIE APALAFLREN LRALHIKFTD IKEIPLWIYS LKTLEELHLT GNLSAENNRY IVIDGLRELK RLKVLRLKSN LSKLPQ VVT DVGVHLQKLS INNEGTKLIV LNSLKKMVNL TELELIRCDL ERIPHSIFSL HNLQEIDLKD NNLKTIEEII SFQHLHR LT CLKLWYNHIA YIPIQIGNLT NLERLYLNRN KIEKIPTQLF YCRKLRYLDL SHNNLTFLPA DIGLLQNLQN LAVTANRI E ALPPELFQCR KLRALHLGNN VLQSLPSRVG ELTNLTQIEL RGNRLECLPV ELGECPLLKR SGLVVEEDLF STLPPEVKE RLWRADKEQA UniProtKB: Volume-regulated anion channel subunit LRRC8A |
-Macromolecule #2: synthetic nanobody Sb5
| Macromolecule | Name: synthetic nanobody Sb5 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 15.463057 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSSSQVQLVE SGGGLVQAGG SLRLSCAASG FPVAQEIMTW YRQAPGKERE WVAAISSIGD TTAYADSVKG RFTISRDNAK NTVYLQMNS LKPEDTAVYY CAVNVGFTYK GQGTQVTVSA GRAGEQKLIS EEDLNSAVDH HHHHH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 67.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)