[English] 日本語
 Yorodumi
Yorodumi- EMDB-12640: In situ subtomogram average of microtubule inner protein from Mus... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12640 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In situ subtomogram average of microtubule inner protein from Mus musculus DRG axons | |||||||||
 Map data Map data | microtubule inner protein structure from mouse DRG axon microtubules | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
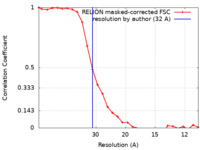
| Method | subtomogram averaging / cryo EM / Resolution: 32.0 Å | |||||||||
 Authors Authors | Foster HE / Ventura Santos C / Carter AP | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: J Cell Biol / Year: 2022 Journal: J Cell Biol / Year: 2022Title: A cryo-ET survey of microtubules and intracellular compartments in mammalian axons. Authors: Helen E Foster / Camilla Ventura Santos / Andrew P Carter /  Abstract: The neuronal axon is packed with cytoskeletal filaments, membranes, and organelles, many of which move between the cell body and axon tip. Here, we used cryo-electron tomography to survey the ...The neuronal axon is packed with cytoskeletal filaments, membranes, and organelles, many of which move between the cell body and axon tip. Here, we used cryo-electron tomography to survey the internal components of mammalian sensory axons. We determined the polarity of the axonal microtubules (MTs) by combining subtomogram classification and visual inspection, finding MT plus and minus ends are structurally similar. Subtomogram averaging of globular densities in the MT lumen suggests they have a defined structure, which is surprising given they likely contain the disordered protein MAP6. We found the endoplasmic reticulum in axons is tethered to MTs through multiple short linkers. We surveyed membrane-bound cargos and describe unexpected internal features such as granules and broken membranes. In addition, we detected proteinaceous compartments, including numerous virus-like capsid particles. Our observations outline novel features of axonal cargos and MTs, providing a platform for identification of their constituents. #1:  Journal: Biorxiv / Year: 2021 Journal: Biorxiv / Year: 2021Title: A cryo-ET survey of intracellular compartments within mammalian axons Authors: Foster HE / Carter AP | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12640.map.gz emd_12640.map.gz | 967.8 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12640-v30.xml emd-12640-v30.xml emd-12640.xml emd-12640.xml | 17.5 KB 17.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12640_fsc.xml emd_12640_fsc.xml | 2.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_12640.png emd_12640.png | 34.4 KB | ||
| Masks |  emd_12640_msk_1.map emd_12640_msk_1.map | 1 MB |  Mask map Mask map | |
| Others |  emd_12640_half_map_1.map.gz emd_12640_half_map_1.map.gz emd_12640_half_map_2.map.gz emd_12640_half_map_2.map.gz | 952.9 KB 953.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12640 http://ftp.pdbj.org/pub/emdb/structures/EMD-12640 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12640 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12640 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10814 (Title: Cryo electron tomograms of mouse DRG axons (dataset 2) EMPIAR-10814 (Title: Cryo electron tomograms of mouse DRG axons (dataset 2)Data size: 206.2 Data #1: Raw image frames of mouse dorsal root ganglion axons (dataset 2) [micrographs - multiframe] Data #2: Corrected, aligned, dose-filtered and order-sorted tilt series for mouse dorsal root ganglion axons (dataset 2) [tilt series] Data #3: Tomograms of mouse dorsal root ganglion axons (dataset 2) binned by 4 and deconvolved [reconstructed volumes]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_12640.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12640.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | microtubule inner protein structure from mouse DRG axon microtubules | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 5.5 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
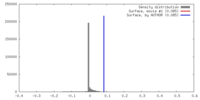
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12640_msk_1.map emd_12640_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
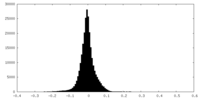
| Density Histograms |
-Half map: half map1
| File | emd_12640_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
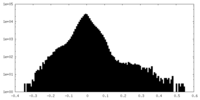
| Density Histograms |
-Half map: half map2
| File | emd_12640_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
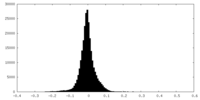
| Density Histograms |
- Sample components
Sample components
-Entire : In situ subtomogram average of microtubule inner protein from Mus...
| Entire | Name: In situ subtomogram average of microtubule inner protein from Mus musculus DRG axons |
|---|---|
| Components |
|
-Supramolecule #1: In situ subtomogram average of microtubule inner protein from Mus...
| Supramolecule | Name: In situ subtomogram average of microtubule inner protein from Mus musculus DRG axons type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Grid | Model: Quantifoil R3.5/1 / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Atmosphere: OTHER Details: Grids were additionally coated in 0.1mg/mL poly-L-lysine then 0.01mg/mL laminin before plating |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 310 K / Instrument: FEI VITROBOT MARK IV / Details: Manual blot for 3 s before plunging. |
| Details | Microtubule inner proteins in axons of adult DRG neurons grown for 7 days in vitro |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3710 pixel / Digitization - Dimensions - Height: 3838 pixel / Digitization - Frames/image: 1-10 / Average exposure time: 1.7 sec. / Average electron dose: 1.85 e/Å2 Details: 61 images per tilt series with 112.85 e/A2 total dose. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 3.0 µm / Nominal magnification: 53000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)