+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12172 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | HRV14 in complex with its receptor ICAM-1 | |||||||||
 Map data Map data | B-factor sharpened (Bf = -82) final map of HRV14-ICAM-1 complex. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | enterovirus / rhinovirus 14 / HRV14 / RV14 / native particle / VIRUS / receptor / virus-receptor complex / ICAM-1 | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of leukocyte mediated cytotoxicity / T cell extravasation / positive regulation of cellular extravasation / regulation of ruffle assembly / T cell antigen processing and presentation / membrane to membrane docking / T cell activation via T cell receptor contact with antigen bound to MHC molecule on antigen presenting cell / lysis of host organelle involved in viral entry into host cell / adhesion of symbiont to host / establishment of endothelial barrier ...regulation of leukocyte mediated cytotoxicity / T cell extravasation / positive regulation of cellular extravasation / regulation of ruffle assembly / T cell antigen processing and presentation / membrane to membrane docking / T cell activation via T cell receptor contact with antigen bound to MHC molecule on antigen presenting cell / lysis of host organelle involved in viral entry into host cell / adhesion of symbiont to host / establishment of endothelial barrier / heterophilic cell-cell adhesion / cell adhesion mediated by integrin / leukocyte migration / leukocyte cell-cell adhesion / Interleukin-10 signaling / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / immunological synapse / Integrin cell surface interactions / negative regulation of endothelial cell apoptotic process / negative regulation of extrinsic apoptotic signaling pathway via death domain receptors / cellular response to leukemia inhibitory factor / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / cellular response to glucose stimulus / host cell cytoplasmic vesicle membrane / : / integrin binding / cellular response to amyloid-beta / Interferon gamma signaling / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / transmembrane signaling receptor activity / nucleoside-triphosphate phosphatase / signaling receptor activity / channel activity / virus receptor activity / Interleukin-4 and Interleukin-13 signaling / monoatomic ion transmembrane transport / DNA replication / receptor-mediated virion attachment to host cell / RNA helicase activity / positive regulation of ERK1 and ERK2 cascade / cell adhesion / membrane raft / endocytosis involved in viral entry into host cell / external side of plasma membrane / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / focal adhesion / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / cell surface / ATP hydrolysis activity / proteolysis / extracellular space / RNA binding / extracellular exosome / zinc ion binding / ATP binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Human rhinovirus 14 / Human rhinovirus 14 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
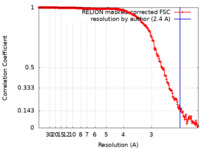
| Method | single particle reconstruction / cryo EM / Resolution: 2.4 Å | |||||||||
 Authors Authors | Hrebik D / Fuzik T | |||||||||
| Funding support |  Czech Republic, 1 items Czech Republic, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: ICAM-1 induced rearrangements of capsid and genome prime rhinovirus 14 for activation and uncoating. Authors: Dominik Hrebík / Tibor Füzik / Mária Gondová / Lenka Šmerdová / Athanassios Adamopoulos / Ondrej Šedo / Zbyněk Zdráhal / Pavel Plevka /  Abstract: Most rhinoviruses, which are the leading cause of the common cold, utilize intercellular adhesion molecule-1 (ICAM-1) as a receptor to infect cells. To release their genomes, rhinoviruses convert to ...Most rhinoviruses, which are the leading cause of the common cold, utilize intercellular adhesion molecule-1 (ICAM-1) as a receptor to infect cells. To release their genomes, rhinoviruses convert to activated particles that contain pores in the capsid, lack minor capsid protein VP4, and have an altered genome organization. The binding of rhinoviruses to ICAM-1 promotes virus activation; however, the molecular details of the process remain unknown. Here, we present the structures of virion of rhinovirus 14 and its complex with ICAM-1 determined to resolutions of 2.6 and 2.4 Å, respectively. The cryo-electron microscopy reconstruction of rhinovirus 14 virions contains the resolved density of octanucleotide segments from the RNA genome that interact with VP2 subunits. We show that the binding of ICAM-1 to rhinovirus 14 is required to prime the virus for activation and genome release at acidic pH. Formation of the rhinovirus 14-ICAM-1 complex induces conformational changes to the rhinovirus 14 capsid, including translocation of the C termini of VP4 subunits, which become poised for release through pores that open in the capsids of activated particles. VP4 subunits with altered conformation block the RNA-VP2 interactions and expose patches of positively charged residues. The conformational changes to the capsid induce the redistribution of the virus genome by altering the capsid-RNA interactions. The restructuring of the rhinovirus 14 capsid and genome prepares the virions for conversion to activated particles. The high-resolution structure of rhinovirus 14 in complex with ICAM-1 explains how the binding of uncoating receptors enables enterovirus genome release. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12172.map.gz emd_12172.map.gz | 164 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12172-v30.xml emd-12172-v30.xml emd-12172.xml emd-12172.xml | 31.3 KB 31.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12172_fsc.xml emd_12172_fsc.xml | 19.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_12172.png emd_12172.png | 310.9 KB | ||
| Masks |  emd_12172_msk_1.map emd_12172_msk_1.map | 209.3 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-12172.cif.gz emd-12172.cif.gz | 7.9 KB | ||
| Others |  emd_12172_additional_1.map.gz emd_12172_additional_1.map.gz emd_12172_additional_2.map.gz emd_12172_additional_2.map.gz emd_12172_half_map_1.map.gz emd_12172_half_map_1.map.gz emd_12172_half_map_2.map.gz emd_12172_half_map_2.map.gz | 135.6 MB 10.2 MB 494.2 MB 494 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12172 http://ftp.pdbj.org/pub/emdb/structures/EMD-12172 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12172 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12172 | HTTPS FTP |
-Related structure data
| Related structure data |  7bg7MC  7bg6C  7nulC  7numC  7nunC  7nuoC  7nuqC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12172.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12172.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B-factor sharpened (Bf = -82) final map of HRV14-ICAM-1 complex. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.063 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
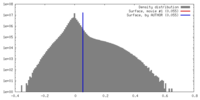
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12172_msk_1.map emd_12172_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
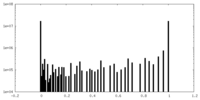
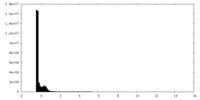
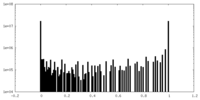
| Density Histograms |
-Additional map: Locally Bfactor sharpened final map of HRV14-ICAM-1 complex...
| File | emd_12172_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Locally Bfactor sharpened final map of HRV14-ICAM-1 complex by LocalDeBlur software. Better visibility of C-terminal part of VP4. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Mask used in final relion postprocess.
| File | emd_12172_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Mask used in final relion postprocess. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Ewald sphere corrected half map 1 used in...
| File | emd_12172_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Ewald sphere corrected half map 1 used in the final relion postprocess. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Ewald sphere corrected half map 2 used in...
| File | emd_12172_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Ewald sphere corrected half map 2 used in the final relion postprocess. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human rhinovirus 14
| Entire | Name:  Human rhinovirus 14 Human rhinovirus 14 |
|---|---|
| Components |
|
-Supramolecule #1: Human rhinovirus 14
| Supramolecule | Name: Human rhinovirus 14 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Molecular weight | Theoretical: 10.0 MDa |
| Virus shell | Shell ID: 1 / Name: HRV14-ICAM-1 complex / Diameter: 325.0 Å / T number (triangulation number): 3 |
-Supramolecule #2: Rhinovirus
| Supramolecule | Name: Rhinovirus / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Human rhinovirus 14 / Strain: 1059 Human rhinovirus 14 / Strain: 1059 |
-Supramolecule #3: Intercellular adhesion molecule 1
| Supramolecule | Name: Intercellular adhesion molecule 1 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Genome polyprotein
| Macromolecule | Name: Genome polyprotein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Human rhinovirus 14 Human rhinovirus 14 |
| Molecular weight | Theoretical: 32.975004 KDa |
| Sequence | String: ALTEGLGDEL EEVIVEKTKQ TVASISSGPK HTQKVPILTA NETGATMPVL PSDSIETRTT YMHFNGSETD VECFLGRAAC VHVTEIQNK DATGIDNHRE AKLFNDWKIN LSSLVQLRKK LELFTYVRFD SEYTILATAS QPDSANYSSN LVVQAMYVPP G APNPKEWD ...String: ALTEGLGDEL EEVIVEKTKQ TVASISSGPK HTQKVPILTA NETGATMPVL PSDSIETRTT YMHFNGSETD VECFLGRAAC VHVTEIQNK DATGIDNHRE AKLFNDWKIN LSSLVQLRKK LELFTYVRFD SEYTILATAS QPDSANYSSN LVVQAMYVPP G APNPKEWD DYTWQSASNP SVFFKVGDTS RFSVPYVGLA SAYNCFYDGY SHDDAETQYG ITVLNHMGSM AFRIVNEHDE HK TLVKIRV YHRAKHVEAW IPRAPRALPY TSIGRTNYPK NTEPVIKKRK GDIKSY UniProtKB: Genome polyprotein |
-Macromolecule #2: Genome polyprotein
| Macromolecule | Name: Genome polyprotein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Human rhinovirus 14 Human rhinovirus 14 |
| Molecular weight | Theoretical: 28.501361 KDa |
| Sequence | String: SPNVEACGYS DRVQQITLGN STITTQEAAN AVVCYAEWPE YLPDVDASDV NKTSKPDTSV CRFYTLDSKT WTTGSKGWCW KLPDALKDM GVFGQNMFFH SLGRSGYTVH VQCNATKFHS GCLLVVVIPE HQLASHEGGN VSVKYTFTHP GERGIDLSSA N EVGGPVKD ...String: SPNVEACGYS DRVQQITLGN STITTQEAAN AVVCYAEWPE YLPDVDASDV NKTSKPDTSV CRFYTLDSKT WTTGSKGWCW KLPDALKDM GVFGQNMFFH SLGRSGYTVH VQCNATKFHS GCLLVVVIPE HQLASHEGGN VSVKYTFTHP GERGIDLSSA N EVGGPVKD VIYNMNGTLL GNLLIFPHQF INLRTNNTAT IVIPYINSVP IDSMTRHNNV SLMVIPIAPL TVPTGATPSL PI TVTIAPM CTEFSGIRSK SIVPQ UniProtKB: Genome polyprotein |
-Macromolecule #3: Genome polyprotein
| Macromolecule | Name: Genome polyprotein / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Human rhinovirus 14 Human rhinovirus 14 |
| Molecular weight | Theoretical: 26.236754 KDa |
| Sequence | String: GLPTTTLPGS GQFLTTDDRQ SPSALPNYEP TPRIHIPGKV HNLLEIIQVD TLIPMNNTHT KDEVNSYLIP LNANRQNEQV FGTNLFIGD GVFKTTLLGE IVQYYTHWSG SLRFSLMYTG PALSSAKLIL AYTPPGARGP QDRREAMLGT HVVWDIGLQS T IVMTIPWT ...String: GLPTTTLPGS GQFLTTDDRQ SPSALPNYEP TPRIHIPGKV HNLLEIIQVD TLIPMNNTHT KDEVNSYLIP LNANRQNEQV FGTNLFIGD GVFKTTLLGE IVQYYTHWSG SLRFSLMYTG PALSSAKLIL AYTPPGARGP QDRREAMLGT HVVWDIGLQS T IVMTIPWT SGVQFRYTDP DTYTSAGFLS CWYQTSLILP PETTGQVYLL SFISACPDFK LRLMKDTQTI SQTVALTE UniProtKB: Genome polyprotein |
-Macromolecule #4: Genome polyprotein
| Macromolecule | Name: Genome polyprotein / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Human rhinovirus 14 Human rhinovirus 14 |
| Molecular weight | Theoretical: 7.183863 KDa |
| Sequence | String: GAQVSTQKSG SHENQNILTN GSNQTFTVIN YYKDAASTSS AGQSLSMDPS KFTEPVKDLM LKGAPALN UniProtKB: Genome polyprotein |
-Macromolecule #5: Intercellular adhesion molecule 1
| Macromolecule | Name: Intercellular adhesion molecule 1 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 49.552004 KDa |
| Recombinant expression | Organism:  Baculovirus expression vector pFastBac1-HM Baculovirus expression vector pFastBac1-HM |
| Sequence | String: QTSVSPSKVI LPRGGSVLVT CSTSCDQPKL LGIETPLPKK ELLLPGNNRK VYELSNVQED SQPMCYSNCP DGQSTAKTFL TVYWTPERV ELAPLPSWQP VGKNLTLRCQ VEGGAPRANL TVVLLRGEKE LKREPAVGEP AEVTTTVLVR RDHHGANFSC R TELDLRPQ ...String: QTSVSPSKVI LPRGGSVLVT CSTSCDQPKL LGIETPLPKK ELLLPGNNRK VYELSNVQED SQPMCYSNCP DGQSTAKTFL TVYWTPERV ELAPLPSWQP VGKNLTLRCQ VEGGAPRANL TVVLLRGEKE LKREPAVGEP AEVTTTVLVR RDHHGANFSC R TELDLRPQ GLELFENTSA PYQLQTFVLP ATPPQLVSPR VLEVDTQGTV VCSLDGLFPV SEAQVHLALG DQRLNPTVTY GN DSFSAKA SVSVTAEDEG TQRLTCAVIL GNQSQETLQT VTIYSFPAPN VILTKPEVSE GTEVTVKCEA HPRAKVTLNG VPA QPLGPR AQLLLKATPE DNGRSFSCSA TLEVAGQLIH KNQTRELRVL YGPRLDERDC PGNWTWPENS QQTPMCQAWG NPLP ELKCL KDGTFPLPIG ESVTVTRDLE GTYLCRARST QGEVTRKVTV NVLSPRYE UniProtKB: Intercellular adhesion molecule 1 |
-Macromolecule #6: water
| Macromolecule | Name: water / type: ligand / ID: 6 / Number of copies: 445 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: PBS | |||||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 3 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 20 sec. / Pretreatment - Atmosphere: OTHER | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 279.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 4680 / Average exposure time: 1.0 sec. / Average electron dose: 83.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus max: 3.5620000000000003 µm / Calibrated defocus min: 0.42 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)