[English] 日本語
 Yorodumi
Yorodumi- EMDB-11738: Cryo-EM structure of human RNA Polymerase III elongation complex 3 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11738 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human RNA Polymerase III elongation complex 3 | |||||||||
 Map data Map data | Cryo-EM map of human RNA Polymerase III, Elongation complex, EC3-Pol III, RPC10 inside funnel, map D, unsharpened | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HUMAN / TRANSCRIPTION / SHORT RNAs | |||||||||
| Function / homology |  Function and homology information Function and homology informationsnRNA transcription by RNA polymerase III / RNA Polymerase III Chain Elongation / DNA/RNA hybrid binding / calcitonin gene-related peptide receptor activity / RNA Polymerase III Transcription Termination / regulation of transcription by RNA polymerase III / regulation of transcription by RNA polymerase I / DNA polymerase III complex / RPAP3/R2TP/prefoldin-like complex / RNA Polymerase III Transcription Initiation From Type 1 Promoter ...snRNA transcription by RNA polymerase III / RNA Polymerase III Chain Elongation / DNA/RNA hybrid binding / calcitonin gene-related peptide receptor activity / RNA Polymerase III Transcription Termination / regulation of transcription by RNA polymerase III / regulation of transcription by RNA polymerase I / DNA polymerase III complex / RPAP3/R2TP/prefoldin-like complex / RNA Polymerase III Transcription Initiation From Type 1 Promoter / RNA Polymerase III Transcription Initiation From Type 2 Promoter / RNA Polymerase III Transcription Initiation From Type 3 Promoter / RNA Polymerase III Abortive And Retractive Initiation / Cytosolic sensors of pathogen-associated DNA / positive regulation of innate immune response / nucleobase-containing compound metabolic process / Abortive elongation of HIV-1 transcript in the absence of Tat / FGFR2 alternative splicing / RNA Polymerase I Transcription Termination / MicroRNA (miRNA) biogenesis / Viral Messenger RNA Synthesis / Signaling by FGFR2 IIIa TM / RNA Pol II CTD phosphorylation and interaction with CE during HIV infection / RNA Pol II CTD phosphorylation and interaction with CE / Formation of the Early Elongation Complex / Formation of the HIV-1 Early Elongation Complex / mRNA Capping / transcription initiation at RNA polymerase III promoter / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / mRNA Splicing - Minor Pathway / PIWI-interacting RNA (piRNA) biogenesis / RNA Polymerase I Transcription Initiation / Processing of Capped Intron-Containing Pre-mRNA / transcription by RNA polymerase III / Pausing and recovery of Tat-mediated HIV elongation / Tat-mediated HIV elongation arrest and recovery / RNA polymerase II transcribes snRNA genes / HIV elongation arrest and recovery / Pausing and recovery of HIV elongation / neuropeptide signaling pathway / Tat-mediated elongation of the HIV-1 transcript / RNA polymerase I complex / RNA polymerase III complex / Formation of HIV-1 elongation complex containing HIV-1 Tat / transcription elongation by RNA polymerase I / Formation of HIV elongation complex in the absence of HIV Tat / RNA polymerase II, core complex / tRNA transcription by RNA polymerase III / transcription by RNA polymerase I / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / RNA Polymerase II Pre-transcription Events / mRNA Splicing - Major Pathway / acrosomal vesicle / positive regulation of interferon-beta production / Inhibition of DNA recombination at telomere / TP53 Regulates Transcription of DNA Repair Genes / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / protein-DNA complex / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / ribonucleoside binding / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Formation of TC-NER Pre-Incision Complex / fibrillar center / DNA-directed RNA polymerase / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / DNA-directed RNA polymerase activity / single-stranded DNA binding / 4 iron, 4 sulfur cluster binding / double-stranded DNA binding / Estrogen-dependent gene expression / defense response to virus / nucleic acid binding / transcription by RNA polymerase II / cell population proliferation / protein dimerization activity / protein stabilization / nuclear body / intracellular membrane-bounded organelle / innate immune response / nucleotide binding / chromatin binding / centrosome / DNA-templated transcription / magnesium ion binding / mitochondrion / DNA binding / zinc ion binding / nucleoplasm / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | |||||||||
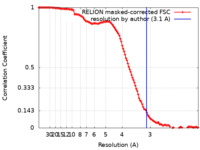
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Girbig M / Misiaszek AD | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Cryo-EM structures of human RNA polymerase III in its unbound and transcribing states. Authors: Mathias Girbig / Agata D Misiaszek / Matthias K Vorländer / Aleix Lafita / Helga Grötsch / Florence Baudin / Alex Bateman / Christoph W Müller /   Abstract: RNA polymerase III (Pol III) synthesizes transfer RNAs and other short, essential RNAs. Human Pol III misregulation is linked to tumor transformation, neurodegenerative and developmental disorders, ...RNA polymerase III (Pol III) synthesizes transfer RNAs and other short, essential RNAs. Human Pol III misregulation is linked to tumor transformation, neurodegenerative and developmental disorders, and increased sensitivity to viral infections. Here, we present cryo-electron microscopy structures at 2.8 to 3.3 Å resolution of transcribing and unbound human Pol III. We observe insertion of the TFIIS-like subunit RPC10 into the polymerase funnel, providing insights into how RPC10 triggers transcription termination. Our structures resolve elements absent from Saccharomyces cerevisiae Pol III such as the winged-helix domains of RPC5 and an iron-sulfur cluster, which tethers the heterotrimer subcomplex to the core. The cancer-associated RPC7α isoform binds the polymerase clamp, potentially interfering with Pol III inhibition by tumor suppressor MAF1, which may explain why overexpressed RPC7α enhances tumor transformation. Finally, the human Pol III structure allows mapping of disease-related mutations and may contribute to the development of inhibitors that selectively target Pol III for therapeutic interventions. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11738.map.gz emd_11738.map.gz | 113.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11738-v30.xml emd-11738-v30.xml emd-11738.xml emd-11738.xml | 45.7 KB 45.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_11738_fsc.xml emd_11738_fsc.xml | 11.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_11738.png emd_11738.png | 92.7 KB | ||
| Masks |  emd_11738_msk_1.map emd_11738_msk_1.map | 144.7 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-11738.cif.gz emd-11738.cif.gz | 11 KB | ||
| Others |  emd_11738_additional_1.map.gz emd_11738_additional_1.map.gz emd_11738_half_map_1.map.gz emd_11738_half_map_1.map.gz emd_11738_half_map_2.map.gz emd_11738_half_map_2.map.gz | 113.9 MB 114.3 MB 114.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11738 http://ftp.pdbj.org/pub/emdb/structures/EMD-11738 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11738 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11738 | HTTPS FTP |
-Related structure data
| Related structure data |  7ae3MC  7a6hC  7ae1C  7aeaC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10697 (Title: Cryo-EM structures of human RNA Polymerase III / Data size: 3.3 TB EMPIAR-10697 (Title: Cryo-EM structures of human RNA Polymerase III / Data size: 3.3 TBData #1: LZW-TIFF compressed multiframe micrographs of human RNA Pol III EC [micrographs - multiframe] Data #2: Initially picked particles of human RNA Pol III EC [picked particles - single frame - processed] Data #3: Polished particles of human RNA Pol III EC (final reconstruction) [picked particles - single frame - processed] Data #4: LZW-TIFF compressed multiframe micrographs of human RNA Pol III apo [micrographs - multiframe] Data #5: Initially picked particles of human RNA Pol III apo [picked particles - single frame - processed] Data #6: Polished particles of human RNA Pol III apo (final reconstruction) [picked particles - single frame - processed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_11738.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11738.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM map of human RNA Polymerase III, Elongation complex, EC3-Pol III, RPC10 inside funnel, map D, unsharpened | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
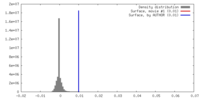
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_11738_msk_1.map emd_11738_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
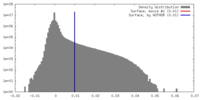
| Density Histograms |
-Additional map: Cryo-EM map of human RNA Polymerase III, Elongation...
| File | emd_11738_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM map of human RNA Polymerase III, Elongation complex, EC3-Pol III, RPC10 inside funnel, map D, locally sharpened via LocalDeblur | ||||||||||||
| Projections & Slices |
| ||||||||||||
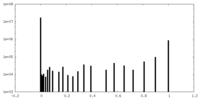
| Density Histograms |
-Half map: #1
| File | emd_11738_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_11738_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
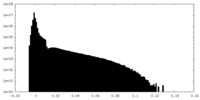
| Density Histograms |
- Sample components
Sample components
+Entire : Human RNA Polymerase III Elongation Complex 3
+Supramolecule #1: Human RNA Polymerase III Elongation Complex 3
+Supramolecule #2: Human RNA Polymerase III Elongation Complex
+Supramolecule #3: RNA, DNA
+Macromolecule #1: DNA-directed RNA polymerase III subunit RPC1
+Macromolecule #2: DNA-directed RNA polymerase III subunit RPC2
+Macromolecule #3: DNA-directed RNA polymerases I and III subunit RPAC1
+Macromolecule #4: DNA-directed RNA polymerase III subunit RPC9
+Macromolecule #5: DNA-directed RNA polymerases I, II, and III subunit RPABC1
+Macromolecule #6: DNA-directed RNA polymerases I, II, and III subunit RPABC2
+Macromolecule #7: DNA-directed RNA polymerase III subunit RPC8
+Macromolecule #8: DNA-directed RNA polymerases I, II, and III subunit RPABC3
+Macromolecule #9: DNA-directed RNA polymerase III subunit RPC10
+Macromolecule #10: DNA-directed RNA polymerases I, II, and III subunit RPABC5
+Macromolecule #11: DNA-directed RNA polymerases I and III subunit RPAC2
+Macromolecule #12: DNA-directed RNA polymerases I, II, and III subunit RPABC4
+Macromolecule #13: DNA-directed RNA polymerase III subunit RPC5
+Macromolecule #14: DNA-directed RNA polymerase III subunit RPC4
+Macromolecule #15: DNA-directed RNA polymerase III subunit RPC3
+Macromolecule #16: DNA-directed RNA polymerase III subunit RPC6
+Macromolecule #17: DNA-directed RNA polymerase III subunit RPC7
+Macromolecule #18: RNA
+Macromolecule #19: Non-template DNA
+Macromolecule #20: Template DNA
+Macromolecule #21: ZINC ION
+Macromolecule #22: IRON/SULFUR CLUSTER
+Macromolecule #23: MAGNESIUM ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Wait time 10 s Blot force 4 Blot time 4 s. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 40.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.75 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)