+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-1136 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | The structure of the poliovirus 135S cell entry intermediate at 10-angstrom resolution reveals the location of an externalized polypeptide that binds to membranes. | |||||||||
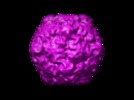
 マップデータ マップデータ | Poliovirus 135S particle produced by heating 160S particles at 50 deg. C for 3 minutes. 135S particles treated with Staphylococcus areus V8 protease. Buffer: 10 mM HEPES, 2 mM CaCl2, 0.07M NaCl, pH 7.5. Images corrected for contrast transfer function and decay before reconstruction. | |||||||||
 試料 試料 |
| |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報symbiont-mediated suppression of host translation initiation / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / ribonucleoside triphosphate phosphatase activity / T=pseudo3 icosahedral viral capsid ...symbiont-mediated suppression of host translation initiation / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / ribonucleoside triphosphate phosphatase activity / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / endocytosis involved in viral entry into host cell / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / RNA helicase activity / induction by virus of host autophagy / RNA-directed RNA polymerase / viral RNA genome replication / cysteine-type endopeptidase activity / RNA-dependent RNA polymerase activity / virus-mediated perturbation of host defense response / DNA-templated transcription / host cell nucleus / virion attachment to host cell / structural molecule activity / proteolysis / RNA binding / ATP binding / membrane / metal ion binding 類似検索 - 分子機能 | |||||||||
| 生物種 |   Human poliovirus 1 Mahoney (ポリオウイルス) Human poliovirus 1 Mahoney (ポリオウイルス) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 8.7 Å | |||||||||
 データ登録者 データ登録者 | Bubeck D / Filman DJ / Cheng N / Steven AC / Hogle JM / Belnap DM | |||||||||
 引用 引用 |  ジャーナル: J Virol / 年: 2005 ジャーナル: J Virol / 年: 2005タイトル: The structure of the poliovirus 135S cell entry intermediate at 10-angstrom resolution reveals the location of an externalized polypeptide that binds to membranes. 著者: Doryen Bubeck / David J Filman / Naiqian Cheng / Alasdair C Steven / James M Hogle / David M Belnap /  要旨: Poliovirus provides a well-characterized system for understanding how nonenveloped viruses enter and infect cells. Upon binding its receptor, poliovirus undergoes an irreversible conformational ...Poliovirus provides a well-characterized system for understanding how nonenveloped viruses enter and infect cells. Upon binding its receptor, poliovirus undergoes an irreversible conformational change to the 135S cell entry intermediate. This transition involves shifts of the capsid protein beta barrels, accompanied by the externalization of VP4 and the N terminus of VP1. Both polypeptides associate with membranes and are postulated to facilitate entry by forming a translocation pore for the viral RNA. We have calculated cryo-electron microscopic reconstructions of 135S particles that permit accurate placement of the beta barrels, loops, and terminal extensions of the capsid proteins. The reconstructions and resulting models indicate that each N terminus of VP1 exits the capsid though an opening in the interface between VP1 and VP3 at the base of the canyon that surrounds the fivefold axis. Comparison with reconstructions of 135S particles in which the first 31 residues of VP1 were proteolytically removed revealed that the externalized N terminus is located near the tips of propeller-like features surrounding the threefold axes rather than at the fivefold axes, as had been proposed in previous models. These observations have forced a reexamination of current models for the role of the 135S particle in transmembrane pore formation and suggest testable alternatives. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_1136.map.gz emd_1136.map.gz | 13.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-1136-v30.xml emd-1136-v30.xml emd-1136.xml emd-1136.xml | 10.2 KB 10.2 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  1136.gif 1136.gif | 43.5 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1136 http://ftp.pdbj.org/pub/emdb/structures/EMD-1136 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1136 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1136 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_1136_validation.pdf.gz emd_1136_validation.pdf.gz | 269.6 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_1136_full_validation.pdf.gz emd_1136_full_validation.pdf.gz | 268.7 KB | 表示 | |
| XML形式データ |  emd_1136_validation.xml.gz emd_1136_validation.xml.gz | 6.4 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1136 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1136 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1136 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1136 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_1136.map.gz / 形式: CCP4 / 大きさ: 28.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_1136.map.gz / 形式: CCP4 / 大きさ: 28.5 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Poliovirus 135S particle produced by heating 160S particles at 50 deg. C for 3 minutes. 135S particles treated with Staphylococcus areus V8 protease. Buffer: 10 mM HEPES, 2 mM CaCl2, 0.07M NaCl, pH 7.5. Images corrected for contrast transfer function and decay before reconstruction. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Poliovirus 135S particle
| 全体 | 名称: Poliovirus 135S particle |
|---|---|
| 要素 |
|
-超分子 #1000: Poliovirus 135S particle
| 超分子 | 名称: Poliovirus 135S particle / タイプ: sample / ID: 1000 詳細: Sedimentation coefficient = 135S. Poliovirus 135S particle produced by heating 160S particles at 50 deg. C for 3 minutes. 集合状態: icosahedrally ordered capsid, 60 copies of VP1, VP2, VP3 Number unique components: 1 |
|---|
-超分子 #1: Human poliovirus 1 Mahoney
| 超分子 | 名称: Human poliovirus 1 Mahoney / タイプ: virus / ID: 1 / Name.synonym: poliovirus / 詳細: 135S particle / NCBI-ID: 12081 / 生物種: Human poliovirus 1 Mahoney / ウイルスタイプ: VIRION / ウイルス・単離状態: STRAIN / ウイルス・エンベロープ: No / ウイルス・中空状態: No / Syn species name: poliovirus |
|---|---|
| 宿主 | 生物種:  Homo sapiens (ヒト) / 別称: VERTEBRATES Homo sapiens (ヒト) / 別称: VERTEBRATES |
| ウイルス殻 | Shell ID: 1 / 名称: capsid / 直径: 339 Å / T番号(三角分割数): 1 |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.5 / 詳細: 20 mM HEPES, 2 mM CaCl2, 0.07 M NaCl |
|---|---|
| 凍結 | 凍結剤: ETHANE 詳細: Vitrification carried out in ambient atmosphere. Ethane cooled by liquid nitrogen. 手法: Blotted manually before plunging |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI/PHILIPS CM200FEG |
|---|---|
| 撮影 | カテゴリ: FILM / フィルム・検出器のモデル: KODAK SO-163 FILM / デジタル化 - スキャナー: ZEISS SCAI / デジタル化 - サンプリング間隔: 7.0 µm / 実像数: 12 / 平均電子線量: 10 e/Å2 / 詳細: Six defocal pairs were scanned. / ビット/ピクセル: 8 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
| 電子線 | 加速電圧: 120 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 38500 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.0 mm / 倍率(公称値): 38000 |
| 試料ステージ | 試料ホルダー: Side entry, liquid nitrogen-cooled, cryo specimen holder 試料ホルダーモデル: GATAN LIQUID NITROGEN |
- 画像解析
画像解析
| 詳細 | Defocal pairs were used. Here, corresponding particle images from each micrograph are counted as one. |
|---|---|
| CTF補正 | 詳細: CTF and decay correction of each particle image |
| 最終 再構成 | 想定した対称性 - 点群: I (正20面体型対称) / アルゴリズム: OTHER / 解像度のタイプ: BY AUTHOR / 解像度: 8.7 Å / 解像度の算出法: FSC 0.33 CUT-OFF / ソフトウェア - 名称: EM3DR2 詳細: Reconstructed computed from focal pairs. Pairs not summed for reconstruction calculaton. 使用した粒子像数: 6850 |
| 最終 角度割当 | 詳細: Determined via PFT2 (used both amplitude and phase information to determine "best" view), focal pairs summed for orientation determination only. |
-原子モデル構築 1
| 詳細 | Protocol: rigid body. see paper for details of the model fitting |
|---|---|
| 精密化 | 空間: RECIPROCAL / プロトコル: RIGID BODY FIT |
| 得られたモデル |  PDB-1xyr: |
 ムービー
ムービー コントローラー
コントローラー
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















