[English] 日本語
 Yorodumi
Yorodumi- EMDB-1133: The structure of the poliovirus 135S cell entry intermediate at 1... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-1133 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
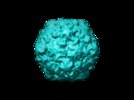
| Title | The structure of the poliovirus 135S cell entry intermediate at 10-angstrom resolution reveals the location of an externalized polypeptide that binds to membranes. | |||||||||
 Map data Map data | Poliovirus 135S particle produced by heating 160S particles at 50 deg. C for 3 minutes. Images corrected for contrast transfer function and decay before reconstruction. | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host translation initiation / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane ...symbiont-mediated suppression of host translation initiation / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of RIG-I activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MDA-5 activity / symbiont-mediated suppression of host cytoplasmic pattern recognition receptor signaling pathway via inhibition of MAVS activity / picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / nucleoside-triphosphate phosphatase / channel activity / monoatomic ion transmembrane transport / RNA helicase activity / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding / membrane Similarity search - Function | |||||||||
| Biological species |   Human poliovirus 1 Mahoney Human poliovirus 1 Mahoney | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 9.6 Å | |||||||||
 Authors Authors | Bubeck D / Filman DJ / Cheng N / Steven AC / Hogle JM / Belnap DM | |||||||||
 Citation Citation |  Journal: J Virol / Year: 2005 Journal: J Virol / Year: 2005Title: The structure of the poliovirus 135S cell entry intermediate at 10-angstrom resolution reveals the location of an externalized polypeptide that binds to membranes. Authors: Doryen Bubeck / David J Filman / Naiqian Cheng / Alasdair C Steven / James M Hogle / David M Belnap /  Abstract: Poliovirus provides a well-characterized system for understanding how nonenveloped viruses enter and infect cells. Upon binding its receptor, poliovirus undergoes an irreversible conformational ...Poliovirus provides a well-characterized system for understanding how nonenveloped viruses enter and infect cells. Upon binding its receptor, poliovirus undergoes an irreversible conformational change to the 135S cell entry intermediate. This transition involves shifts of the capsid protein beta barrels, accompanied by the externalization of VP4 and the N terminus of VP1. Both polypeptides associate with membranes and are postulated to facilitate entry by forming a translocation pore for the viral RNA. We have calculated cryo-electron microscopic reconstructions of 135S particles that permit accurate placement of the beta barrels, loops, and terminal extensions of the capsid proteins. The reconstructions and resulting models indicate that each N terminus of VP1 exits the capsid though an opening in the interface between VP1 and VP3 at the base of the canyon that surrounds the fivefold axis. Comparison with reconstructions of 135S particles in which the first 31 residues of VP1 were proteolytically removed revealed that the externalized N terminus is located near the tips of propeller-like features surrounding the threefold axes rather than at the fivefold axes, as had been proposed in previous models. These observations have forced a reexamination of current models for the role of the 135S particle in transmembrane pore formation and suggest testable alternatives. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_1133.map.gz emd_1133.map.gz | 13.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-1133-v30.xml emd-1133-v30.xml emd-1133.xml emd-1133.xml | 10.1 KB 10.1 KB | Display Display |  EMDB header EMDB header |
| Images |  1133.gif 1133.gif | 34.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1133 http://ftp.pdbj.org/pub/emdb/structures/EMD-1133 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1133 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1133 | HTTPS FTP |
-Related structure data
| Related structure data |  1xyrMC  1136C  1137C  1144C  1145C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_1133.map.gz / Format: CCP4 / Size: 28.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_1133.map.gz / Format: CCP4 / Size: 28.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Poliovirus 135S particle produced by heating 160S particles at 50 deg. C for 3 minutes. Images corrected for contrast transfer function and decay before reconstruction. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.816 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Poliovirus 135S particle
| Entire | Name: Poliovirus 135S particle |
|---|---|
| Components |
|
-Supramolecule #1000: Poliovirus 135S particle
| Supramolecule | Name: Poliovirus 135S particle / type: sample / ID: 1000 Details: Sedimentation coefficient = 135S. Poliovirus 135S particle produced by heating 160S particles at 50 deg. C for 3 minutes. Oligomeric state: icosahedrally ordered capsid, 60 copies of VP1, VP2, VP3 Number unique components: 1 |
|---|
-Supramolecule #1: Human poliovirus 1 Mahoney
| Supramolecule | Name: Human poliovirus 1 Mahoney / type: virus / ID: 1 / Name.synonym: poliovirus / Details: 135S particle / NCBI-ID: 12081 / Sci species name: Human poliovirus 1 Mahoney / Virus type: VIRION / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No / Syn species name: poliovirus |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) / synonym: VERTEBRATES Homo sapiens (human) / synonym: VERTEBRATES |
| Virus shell | Shell ID: 1 / Name: capsid / Diameter: 339 Å / T number (triangulation number): 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 / Details: 20 mM Tris, 2 mM CaCl2 |
|---|---|
| Vitrification | Cryogen name: ETHANE Details: Vitrification carried out in ambient atmosphere. Ethane cooled by liquid nitrogen. Method: Blotted manually before plunging |
- Electron microscopy
Electron microscopy
| Microscope | FEI/PHILIPS CM200FEG |
|---|---|
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: ZEISS SCAI / Digitization - Sampling interval: 7 µm / Number real images: 6 / Average electron dose: 10 e/Å2 Details: Defocal pairs were used. Here, corresponding particle images from each micrograph are counted as one. Three focal pairs were scanned. Bits/pixel: 8 |
| Tilt angle min | 0 |
| Tilt angle max | 0 |
| Electron beam | Acceleration voltage: 120 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 38500 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2 mm / Nominal magnification: 38000 |
| Sample stage | Specimen holder: Side entry liquid nitrogen-cooled cryo specimen holder Specimen holder model: GATAN LIQUID NITROGEN |
- Image processing
Image processing
| Details | Poliovirus 135S particle produced by heating 160S particles at 50 deg. C for 3 minutes. |
|---|---|
| CTF correction | Details: CTF and decay correction of each particle |
| Final reconstruction | Applied symmetry - Point group: I (icosahedral) / Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 9.6 Å / Resolution method: FSC 0.33 CUT-OFF / Software - Name: EM3DR2 Details: Reconstructed computed from focal pairs. Pairs not summed for reconstruction calculaton. Number images used: 8224 |
| Final angle assignment | Details: Determined via PFT2 using both amplitude and phase information to determine best view. Focal pairs summed for orientation determination only. |
-Atomic model buiding 1
| Details | Protocol: rigid body. see paper for details of the model fitting |
|---|---|
| Refinement | Space: RECIPROCAL / Protocol: RIGID BODY FIT |
| Output model |  PDB-1xyr: |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















