[English] 日本語
 Yorodumi
Yorodumi- EMDB-10292: Structure of the Fanconi Anaemia core complex (focussed map for m... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10292 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the Fanconi Anaemia core complex (focussed map for middle region) | |||||||||
 Map data Map data | Post processed map for the middle region. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
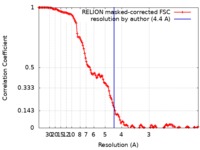
| Method | single particle reconstruction / cryo EM / Resolution: 4.4 Å | |||||||||
 Authors Authors | Shakeel S / Rajendra E / Alcon P / He S / Scheres SHW / Passmore LA | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Structure of the Fanconi anaemia monoubiquitin ligase complex. Authors: Shabih Shakeel / Eeson Rajendra / Pablo Alcón / Francis O'Reilly / Dror S Chorev / Sarah Maslen / Gianluca Degliesposti / Christopher J Russo / Shaoda He / Chris H Hill / J Mark Skehel / ...Authors: Shabih Shakeel / Eeson Rajendra / Pablo Alcón / Francis O'Reilly / Dror S Chorev / Sarah Maslen / Gianluca Degliesposti / Christopher J Russo / Shaoda He / Chris H Hill / J Mark Skehel / Sjors H W Scheres / Ketan J Patel / Juri Rappsilber / Carol V Robinson / Lori A Passmore /   Abstract: The Fanconi anaemia (FA) pathway repairs DNA damage caused by endogenous and chemotherapy-induced DNA crosslinks, and responds to replication stress. Genetic inactivation of this pathway by mutation ...The Fanconi anaemia (FA) pathway repairs DNA damage caused by endogenous and chemotherapy-induced DNA crosslinks, and responds to replication stress. Genetic inactivation of this pathway by mutation of genes encoding FA complementation group (FANC) proteins impairs development, prevents blood production and promotes cancer. The key molecular step in the FA pathway is the monoubiquitination of a pseudosymmetric heterodimer of FANCD2-FANCI by the FA core complex-a megadalton multiprotein E3 ubiquitin ligase. Monoubiquitinated FANCD2 then recruits additional protein factors to remove the DNA crosslink or to stabilize the stalled replication fork. A molecular structure of the FA core complex would explain how it acts to maintain genome stability. Here we reconstituted an active, recombinant FA core complex, and used cryo-electron microscopy and mass spectrometry to determine its structure. The FA core complex comprises two central dimers of the FANCB and FA-associated protein of 100 kDa (FAAP100) subunits, flanked by two copies of the RING finger subunit, FANCL. These two heterotrimers act as a scaffold to assemble the remaining five subunits, resulting in an extended asymmetric structure. Destabilization of the scaffold would disrupt the entire complex, resulting in a non-functional FA pathway. Thus, the structure provides a mechanistic basis for the low numbers of patients with mutations in FANCB, FANCL and FAAP100. Despite a lack of sequence homology, FANCB and FAAP100 adopt similar structures. The two FANCL subunits are in different conformations at opposite ends of the complex, suggesting that each FANCL has a distinct role. This structural and functional asymmetry of dimeric RING finger domains may be a general feature of E3 ligases. The cryo-electron microscopy structure of the FA core complex provides a foundation for a detailed understanding of its E3 ubiquitin ligase activity and DNA interstrand crosslink repair. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10292.map.gz emd_10292.map.gz | 13.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10292-v30.xml emd-10292-v30.xml emd-10292.xml emd-10292.xml | 22.6 KB 22.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10292_fsc.xml emd_10292_fsc.xml | 13.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_10292.png emd_10292.png | 93.9 KB | ||
| Masks |  emd_10292_msk_1.map emd_10292_msk_1.map | 209.3 MB |  Mask map Mask map | |
| Others |  emd_10292_additional.map.gz emd_10292_additional.map.gz emd_10292_half_map_1.map.gz emd_10292_half_map_1.map.gz emd_10292_half_map_2.map.gz emd_10292_half_map_2.map.gz | 156.4 MB 158.1 MB 158.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10292 http://ftp.pdbj.org/pub/emdb/structures/EMD-10292 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10292 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10292 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10292.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10292.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Post processed map for the middle region. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10292_msk_1.map emd_10292_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
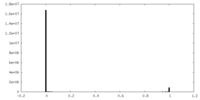
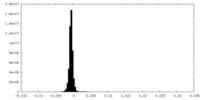
| Density Histograms |
-Additional map: This is the map from auto refinement in Relion.
| File | emd_10292_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This is the map from auto refinement in Relion. | ||||||||||||
| Projections & Slices |
| ||||||||||||
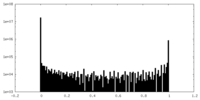
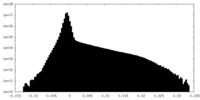
| Density Histograms |
-Half map: This is half1 map from auto refinement in Relion.
| File | emd_10292_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This is half1 map from auto refinement in Relion. | ||||||||||||
| Projections & Slices |
| ||||||||||||
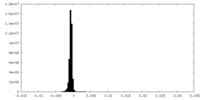
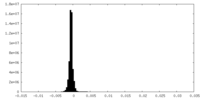
| Density Histograms |
-Half map: This is half2 map from auto refinement in Relion.
| File | emd_10292_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This is half2 map from auto refinement in Relion. | ||||||||||||
| Projections & Slices |
| ||||||||||||
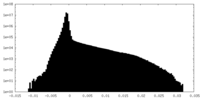
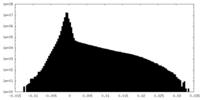
| Density Histograms |
- Sample components
Sample components
-Entire : Fanconi anaemia core complex
| Entire | Name: Fanconi anaemia core complex |
|---|---|
| Components |
|
-Supramolecule #1: Fanconi anaemia core complex
| Supramolecule | Name: Fanconi anaemia core complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#28 |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 860 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL |
|---|---|
| Buffer | pH: 8 / Details: 50 mM HEPES pH 8.0, ~500 mM NaCl, 1 mM TCEP |
| Grid | Model: Quantifoil, UltrAuFoil, R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV Details: Blot time: 3 to 4.5 s Wait time: 0 s Drain time: 0 s Force: -10 N. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Number real images: 4145 / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -4.0 µm / Nominal defocus min: -1.8 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: OTHER |
|---|
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)