[English] 日本語
 Yorodumi
Yorodumi- EMDB-10220: Cryo-EM structure of rhinovirus-B5 complexed to antiviral OBR-5-340 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10220 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of rhinovirus-B5 complexed to antiviral OBR-5-340 | |||||||||
 Map data Map data | None | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | RV-A89 / HRV-B5 / virus / capsid protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationpicornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / viral capsid / nucleoside-triphosphate phosphatase / host cell ...picornain 2A / symbiont-mediated suppression of host mRNA export from nucleus / symbiont genome entry into host cell via pore formation in plasma membrane / picornain 3C / T=pseudo3 icosahedral viral capsid / host cell cytoplasmic vesicle membrane / ribonucleoside triphosphate phosphatase activity / viral capsid / nucleoside-triphosphate phosphatase / host cell / channel activity / monoatomic ion transmembrane transport / RNA helicase activity / symbiont-mediated suppression of host innate immune response / endocytosis involved in viral entry into host cell / symbiont-mediated activation of host autophagy / RNA-directed RNA polymerase / cysteine-type endopeptidase activity / viral RNA genome replication / RNA-directed RNA polymerase activity / symbiont entry into host cell / DNA-templated transcription / virion attachment to host cell / host cell nucleus / structural molecule activity / proteolysis / RNA binding / zinc ion binding / ATP binding Similarity search - Function | |||||||||
| Biological species |  Human rhinovirus B5 Human rhinovirus B5 | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||
 Authors Authors | Wald J / Goessweiner-Mohr N | |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2019 Journal: Proc Natl Acad Sci U S A / Year: 2019Title: Cryo-EM structure of pleconaril-resistant rhinovirus-B5 complexed to the antiviral OBR-5-340 reveals unexpected binding site. Authors: Jiri Wald / Marion Pasin / Martina Richter / Christin Walther / Neann Mathai / Johannes Kirchmair / Vadim A Makarov / Nikolaus Goessweiner-Mohr / Thomas C Marlovits / Irene Zanella / Antonio ...Authors: Jiri Wald / Marion Pasin / Martina Richter / Christin Walther / Neann Mathai / Johannes Kirchmair / Vadim A Makarov / Nikolaus Goessweiner-Mohr / Thomas C Marlovits / Irene Zanella / Antonio Real-Hohn / Nuria Verdaguer / Dieter Blaas / Michaela Schmidtke /      Abstract: Viral inhibitors, such as pleconaril and vapendavir, target conserved regions in the capsids of rhinoviruses (RVs) and enteroviruses (EVs) by binding to a hydrophobic pocket in viral capsid protein 1 ...Viral inhibitors, such as pleconaril and vapendavir, target conserved regions in the capsids of rhinoviruses (RVs) and enteroviruses (EVs) by binding to a hydrophobic pocket in viral capsid protein 1 (VP1). In resistant RVs and EVs, bulky residues in this pocket prevent their binding. However, recently developed pyrazolopyrimidines inhibit pleconaril-resistant RVs and EVs, and computational modeling has suggested that they also bind to the hydrophobic pocket in VP1. We studied the mechanism of inhibition of pleconaril-resistant RVs using RV-B5 (1 of the 7 naturally pleconaril-resistant rhinoviruses) and OBR-5-340, a bioavailable pyrazolopyrimidine with proven in vivo activity, and determined the 3D-structure of the protein-ligand complex to 3.6 Å with cryoelectron microscopy. Our data indicate that, similar to other capsid binders, OBR-5-340 induces thermostability and inhibits viral adsorption and uncoating. However, we found that OBR-5-340 attaches closer to the entrance of the pocket than most other capsid binders, whose viral complexes have been studied so far, showing only marginal overlaps of the attachment sites. Comparing the experimentally determined 3D structure with the control, RV-B5 incubated with solvent only and determined to 3.2 Å, revealed no gross conformational changes upon OBR-5-340 binding. The pocket of the naturally OBR-5-340-resistant RV-A89 likewise incubated with OBR-5-340 and solved to 2.9 Å was empty. Pyrazolopyrimidines have a rigid molecular scaffold and may thus be less affected by a loss of entropy upon binding. They interact with less-conserved regions than known capsid binders. Overall, pyrazolopyrimidines could be more suitable for the development of new, broadly active inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10220.map.gz emd_10220.map.gz | 74 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10220-v30.xml emd-10220-v30.xml emd-10220.xml emd-10220.xml | 19.5 KB 19.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_10220.png emd_10220.png | 341.8 KB | ||
| Masks |  emd_10220_msk_1.map emd_10220_msk_1.map | 347.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-10220.cif.gz emd-10220.cif.gz | 6.6 KB | ||
| Others |  emd_10220_half_map_1.map.gz emd_10220_half_map_1.map.gz emd_10220_half_map_2.map.gz emd_10220_half_map_2.map.gz | 276.1 MB 276.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10220 http://ftp.pdbj.org/pub/emdb/structures/EMD-10220 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10220 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10220 | HTTPS FTP |
-Related structure data
| Related structure data |  6sk5MC  6sk6C  6sk7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10220.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10220.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | None | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.97 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
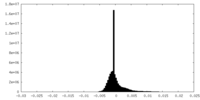
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10220_msk_1.map emd_10220_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
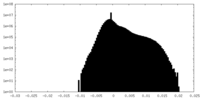
| Density Histograms |
-Half map: #2
| File | emd_10220_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_10220_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human rhinovirus B5
| Entire | Name:  Human rhinovirus B5 Human rhinovirus B5 |
|---|---|
| Components |
|
-Supramolecule #1: Human rhinovirus B5
| Supramolecule | Name: Human rhinovirus B5 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 / NCBI-ID: 147714 / Sci species name: Human rhinovirus B5 / Sci species strain: B5 / Virus type: VIRION / Virus isolate: SEROTYPE / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Virus shell | Shell ID: 1 / Name: VP1-4 / Diameter: 302.0 Å |
-Macromolecule #1: Rhinovirus B5 VP4
| Macromolecule | Name: Rhinovirus B5 VP4 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human rhinovirus B5 Human rhinovirus B5 |
| Molecular weight | Theoretical: 7.335049 KDa |
| Sequence | String: MGAQVSTQKS GSHENQNILT NGSNQTFTVI NYYKDAASSS SAGQSFSMDP SKFTEPVKDI MLKGAPALN UniProtKB: VP4 |
-Macromolecule #2: Rhinovirus B5 VP2
| Macromolecule | Name: Rhinovirus B5 VP2 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Human rhinovirus B5 Human rhinovirus B5 |
| Molecular weight | Theoretical: 27.507256 KDa |
| Sequence | String: GYSDRVEQIT LGNSTITTQE AANSIVAYGE WPSFLSDVDA SDVNKTTKPD TSACRFYTLD SKMWTQGSKG WCWKLPDALK DMGIFGQNM FFHSQGRTGY TIHVQCNATK FHSGCLLVVV IPEHQLASAE GGNVSVLYDK THPGEKGIDL SEADSTGPMK D PLYMMDGT ...String: GYSDRVEQIT LGNSTITTQE AANSIVAYGE WPSFLSDVDA SDVNKTTKPD TSACRFYTLD SKMWTQGSKG WCWKLPDALK DMGIFGQNM FFHSQGRTGY TIHVQCNATK FHSGCLLVVV IPEHQLASAE GGNVSVLYDK THPGEKGIDL SEADSTGPMK D PLYMMDGT LIGNSLIFPH QFINLRTNNT ATIVVPYINS VPMDSMTRHN NLSLMVIPIV DITATSGTTP SIPVTITIAP MF LELSGIR SKAVI UniProtKB: Genome polyprotein |
-Macromolecule #3: Rhinovirus B5 VP1
| Macromolecule | Name: Rhinovirus B5 VP1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Human rhinovirus B5 Human rhinovirus B5 |
| Molecular weight | Theoretical: 32.743861 KDa |
| Sequence | String: GLEDDLVEVI VDKAQQTLAS IKSDSKHTQK VPSLTANETG ATLPTTPSDS VETRTTLMHY TGSETTLENF LGRAACVHVV EIVNKRPTD TEEHRMQLLF NNWKINLSSL VQLRRKLEMF TYVRFDSEYT IIATSSQPNE AKFSSNLTIQ AMFIPPGAPN P KKWDDYTW ...String: GLEDDLVEVI VDKAQQTLAS IKSDSKHTQK VPSLTANETG ATLPTTPSDS VETRTTLMHY TGSETTLENF LGRAACVHVV EIVNKRPTD TEEHRMQLLF NNWKINLSSL VQLRRKLEMF TYVRFDSEYT IIATSSQPNE AKFSSNLTIQ AMFIPPGAPN P KKWDDYTW QSATNPSVFF NVGKSARFSV PYLGIASAYN CFYDGYSHDN STTPYGINVL NHMGSMAFRV VNEHDNHTTH VK VRVYHRA KHIRAWVPRA PRALEYLHIG RTNYKQSPQN PIKTRKTIST Y UniProtKB: Genome polyprotein |
-Macromolecule #4: Rhinovirus B5 VP3
| Macromolecule | Name: Rhinovirus B5 VP3 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO / EC number: picornain 2A |
|---|---|
| Source (natural) | Organism:  Human rhinovirus B5 Human rhinovirus B5 |
| Molecular weight | Theoretical: 25.500168 KDa |
| Sequence | String: GLPTVLTPGS EQFLTTDDRQ SPSAMPNYEP TPLIHIPGEV KNLLEIAQVD TLIPLNNTTN TTGLGMYRIP LVQNMQGEQV FGFRLYLGD GVLKTTLLGE LCQYFTHWAG SLRLSFMYTG PALSSAKLLI AYTPPGAQGP TKRKEAMLGT HVVWDIGLQS T VVLNIPWT ...String: GLPTVLTPGS EQFLTTDDRQ SPSAMPNYEP TPLIHIPGEV KNLLEIAQVD TLIPLNNTTN TTGLGMYRIP LVQNMQGEQV FGFRLYLGD GVLKTTLLGE LCQYFTHWAG SLRLSFMYTG PALSSAKLLI AYTPPGAQGP TKRKEAMLGT HVVWDIGLQS T VVLNIPWT SGVQYRYTDP DTYTSAGFVS CWYQTSLVLP PQTQQTVYML GFISACPDFK LRLMKDTQSI HQ UniProtKB: Genome polyprotein |
-Macromolecule #5: 6-phenyl-~{N}3-[4-(trifluoromethyl)phenyl]-1~{H}-pyrazolo[3,4-d]p...
| Macromolecule | Name: 6-phenyl-~{N}3-[4-(trifluoromethyl)phenyl]-1~{H}-pyrazolo[3,4-d]pyrimidine-3,4-diamine type: ligand / ID: 5 / Number of copies: 1 / Formula: LGQ |
|---|---|
| Molecular weight | Theoretical: 370.331 Da |
| Chemical component information | 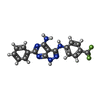 ChemComp-LGQ: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 / Component:
| ||||||
|---|---|---|---|---|---|---|---|
| Grid | Model: Homemade / Material: COPPER / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 0.4 / Pretreatment - Type: GLOW DISCHARGE | ||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK III |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-25 / Number real images: 2547 / Average exposure time: 5.0 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C60 (60 fold cyclic) / Resolution.type: BY AUTHOR / Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 46070 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Details | Rosetta: DiMaio, F. et al. Atomic-accuracy models from 4.5-A cryo-electron microscopy data with density-guided iterative local refinement. Nat. Meth (2015). doi:10.1038/nmeth.3286 |
| Refinement | Space: REAL |
| Output model |  PDB-6sk5: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)