+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0758 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
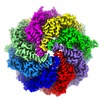
| Title | TRiC at 0.2 mM ADP-AlFx, Conformation 1, 0.2-C1 | |||||||||||||||
 Map data Map data | TRiC at 0.2 mM ADP-AlFx, Conformation 1, 0.2-C1 | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Chaperonin TRiC/CCT / Allosteric network / ATPase cycle / Conformational landscape / Cryo-EM / CHAPERONE | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationAssociation of TriC/CCT with target proteins during biosynthesis / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / : / chaperonin-containing T-complex / Neutrophil degranulation / ATP-dependent protein folding chaperone / unfolded protein binding / protein folding / ATP hydrolysis activity / ATP binding ...Association of TriC/CCT with target proteins during biosynthesis / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / : / chaperonin-containing T-complex / Neutrophil degranulation / ATP-dependent protein folding chaperone / unfolded protein binding / protein folding / ATP hydrolysis activity / ATP binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.99 Å | |||||||||||||||
 Authors Authors | Jin M / Cong Y | |||||||||||||||
| Funding support |  China, 4 items China, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2019 Journal: Proc Natl Acad Sci U S A / Year: 2019Title: An ensemble of cryo-EM structures of TRiC reveal its conformational landscape and subunit specificity. Authors: Mingliang Jin / Wenyu Han / Caixuan Liu / Yunxiang Zang / Jiawei Li / Fangfang Wang / Yanxing Wang / Yao Cong /  Abstract: TRiC/CCT assists the folding of ∼10% of cytosolic proteins through an ATP-driven conformational cycle and is essential in maintaining protein homeostasis. Here, we determined an ensemble of cryo- ...TRiC/CCT assists the folding of ∼10% of cytosolic proteins through an ATP-driven conformational cycle and is essential in maintaining protein homeostasis. Here, we determined an ensemble of cryo-electron microscopy (cryo-EM) structures of yeast TRiC at various nucleotide concentrations, with 4 open-state maps resolved at near-atomic resolutions, and a closed-state map at atomic resolution, revealing an extra layer of an unforeseen N-terminal allosteric network. We found that, during TRiC ring closure, the CCT7 subunit moves first, responding to nucleotide binding; CCT4 is the last to bind ATP, serving as an ATP sensor; and CCT8 remains ADP-bound and is hardly involved in the ATPase-cycle in our experimental conditions; overall, yeast TRiC consumes nucleotide in a 2-ring positively coordinated manner. Our results depict a thorough picture of the TRiC conformational landscape and its allosteric transitions from the open to closed states in more structural detail and offer insights into TRiC subunit specificity in ATP consumption and ring closure, and potentially in substrate processing. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0758.map.gz emd_0758.map.gz | 23.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0758-v30.xml emd-0758-v30.xml emd-0758.xml emd-0758.xml | 29 KB 29 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0758.png emd_0758.png | 195.9 KB | ||
| Filedesc metadata |  emd-0758.cif.gz emd-0758.cif.gz | 9.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0758 http://ftp.pdbj.org/pub/emdb/structures/EMD-0758 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0758 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0758 | HTTPS FTP |
-Related structure data
| Related structure data |  6ks6MC  0756C  0757C  0759C  0760C  0761C  0762C  0763C  0764C  0765C  0766C  0767C  6krdC  6kreC  6ks7C  6ks8C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0758.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0758.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TRiC at 0.2 mM ADP-AlFx, Conformation 1, 0.2-C1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.659 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : TRiC complex
+Supramolecule #1: TRiC complex
+Macromolecule #1: T-complex protein 1 subunit alpha
+Macromolecule #2: T-complex protein 1 subunit beta
+Macromolecule #3: T-complex protein 1 subunit delta
+Macromolecule #4: T-complex protein 1 subunit epsilon
+Macromolecule #5: fusion protein of T-complex protein 1 subunit gamma,rep-His-CBP a...
+Macromolecule #6: T-complex protein 1 subunit eta
+Macromolecule #7: T-complex protein 1 subunit theta
+Macromolecule #8: T-complex protein 1 subunit zeta
+Macromolecule #9: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #10: MAGNESIUM ION
+Macromolecule #11: ALUMINUM FLUORIDE
+Macromolecule #12: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 38.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)

























