[English] 日本語
 Yorodumi
Yorodumi- EMDB-0664: Electron cryo-microscopy of the eukaryotic translation initiation... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0664 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
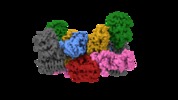
| Title | Electron cryo-microscopy of the eukaryotic translation initiation factor 2B bound to eukaryotic translation initiation factor 2 from Homo sapiens | |||||||||
 Map data Map data | eIF2 alpha phosphorylated at serine 51 bound to eIF2B. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | eukaryotic translation initiation factor 2B / eukaryotic translation initiation factor 2 / TRANSLATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationtranslation initiation ternary complex / regulation of translation in response to endoplasmic reticulum stress / glial limiting end-foot / HRI-mediated signaling / eukaryotic translation initiation factor 2B complex / response to manganese-induced endoplasmic reticulum stress / Cellular response to mitochondrial stress / positive regulation of type B pancreatic cell apoptotic process / Response of EIF2AK1 (HRI) to heme deficiency / Recycling of eIF2:GDP ...translation initiation ternary complex / regulation of translation in response to endoplasmic reticulum stress / glial limiting end-foot / HRI-mediated signaling / eukaryotic translation initiation factor 2B complex / response to manganese-induced endoplasmic reticulum stress / Cellular response to mitochondrial stress / positive regulation of type B pancreatic cell apoptotic process / Response of EIF2AK1 (HRI) to heme deficiency / Recycling of eIF2:GDP / negative regulation of translational initiation in response to stress / PERK-mediated unfolded protein response / PERK regulates gene expression / response to kainic acid / eukaryotic translation initiation factor 2 complex / cytoplasmic translational initiation / regulation of translational initiation in response to stress / astrocyte development / guanyl-nucleotide exchange factor complex / eukaryotic 48S preinitiation complex / astrocyte differentiation / oligodendrocyte development / regulation of translational initiation / Formation of the ternary complex, and subsequently, the 43S complex / Ribosomal scanning and start codon recognition / Translation initiation complex formation / positive regulation of translational initiation / Response of EIF2AK4 (GCN2) to amino acid deficiency / response to glucose / GTP hydrolysis and joining of the 60S ribosomal subunit / L13a-mediated translational silencing of Ceruloplasmin expression / ovarian follicle development / mitophagy / myelination / translation initiation factor binding / translation initiation factor activity / stress granule assembly / guanyl-nucleotide exchange factor activity / cellular response to amino acid starvation / response to endoplasmic reticulum stress / central nervous system development / hippocampus development / translational initiation / PKR-mediated signaling / ABC-family proteins mediated transport / response to peptide hormone / cytoplasmic stress granule / cellular response to UV / T cell receptor signaling pathway / regulation of translation / cellular response to heat / ribosome binding / response to heat / cellular response to oxidative stress / positive regulation of apoptotic process / synapse / GTP binding / mitochondrion / RNA binding / extracellular exosome / ATP binding / identical protein binding / membrane / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.03 Å | |||||||||
 Authors Authors | Nguyen HC / Kenner LR | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: eIF2B-catalyzed nucleotide exchange and phosphoregulation by the integrated stress response. Authors: Lillian R Kenner / Aditya A Anand / Henry C Nguyen / Alexander G Myasnikov / Carolin J Klose / Lea A McGeever / Jordan C Tsai / Lakshmi E Miller-Vedam / Peter Walter / Adam Frost /   Abstract: The integrated stress response (ISR) tunes the rate of protein synthesis. Control is exerted by phosphorylation of the general translation initiation factor eIF2. eIF2 is a guanosine triphosphatase ...The integrated stress response (ISR) tunes the rate of protein synthesis. Control is exerted by phosphorylation of the general translation initiation factor eIF2. eIF2 is a guanosine triphosphatase that becomes activated by eIF2B, a two-fold symmetric and heterodecameric complex that functions as eIF2's dedicated nucleotide exchange factor. Phosphorylation converts eIF2 from a substrate into an inhibitor of eIF2B. We report cryo-electron microscopy structures of eIF2 bound to eIF2B in the dephosphorylated state. The structures reveal that the eIF2B decamer is a static platform upon which one or two flexible eIF2 trimers bind and align with eIF2B's bipartite catalytic centers to catalyze nucleotide exchange. Phosphorylation refolds eIF2α, allowing it to contact eIF2B at a different interface and, we surmise, thereby sequestering it into a nonproductive complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0664.map.gz emd_0664.map.gz | 154.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0664-v30.xml emd-0664-v30.xml emd-0664.xml emd-0664.xml | 21.2 KB 21.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0664.png emd_0664.png | 464.2 KB | ||
| Filedesc metadata |  emd-0664.cif.gz emd-0664.cif.gz | 8.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0664 http://ftp.pdbj.org/pub/emdb/structures/EMD-0664 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0664 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0664 | HTTPS FTP |
-Related structure data
| Related structure data |  6o9zMC  0649C  0651C  6o81C  6o85C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0664.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0664.map.gz / Format: CCP4 / Size: 166.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | eIF2 alpha phosphorylated at serine 51 bound to eIF2B. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.822 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : eIF2 Alpha-P bound to eIF2B
| Entire | Name: eIF2 Alpha-P bound to eIF2B |
|---|---|
| Components |
|
-Supramolecule #1: eIF2 Alpha-P bound to eIF2B
| Supramolecule | Name: eIF2 Alpha-P bound to eIF2B / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Translation initiation factor eIF-2B subunit epsilon
| Macromolecule | Name: Translation initiation factor eIF-2B subunit epsilon / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 80.452586 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAAPVVAPPG VVVSRANKRS GAGPGGSGGG GARGAEEEPP PPLQAVLVAD SFDRRFFPIS KDQPRVLLPL ANVALIDYTL EFLTATGVQ ETFVFCCWKA AQIKEHLLKS KWCRPTSLNV VRIITSELYR SLGDVLRDVD AKALVRSDFL LVYGDVISNI N ITRALEEH ...String: MAAPVVAPPG VVVSRANKRS GAGPGGSGGG GARGAEEEPP PPLQAVLVAD SFDRRFFPIS KDQPRVLLPL ANVALIDYTL EFLTATGVQ ETFVFCCWKA AQIKEHLLKS KWCRPTSLNV VRIITSELYR SLGDVLRDVD AKALVRSDFL LVYGDVISNI N ITRALEEH RLRRKLEKNV SVMTMIFKES SPSHPTRCHE DNVVVAVDST TNRVLHFQKT QGLRRFAFPL SLFQGSSDGV EV RYDLLDC HISICSPQVA QLFTDNFDYQ TRDDFVRGLL VNEEILGNQI HMHVTAKEYG ARVSNLHMYS AVCADVIRRW VYP LTPEAN FTDSTTQSCT HSRHNIYRGP EVSLGHGSIL EENVLLGSGT VIGSNCFITN SVIGPGCHIG DNVVLDQTYL WQGV RVAAG AQIHQSLLCD NAEVKERVTL KPRSVLTSQV VVGPNITLPE GSVISLHPPD AEEDEDDGEF SDDSGADQEK DKVKM KGYN PAEVGAAGKG YLWKAAGMNM EEEEELQQNL WGLKINMEEE SESESEQSMD SEEPDSRGGS PQMDDIKVFQ NEVLGT LQR GKEENISCDN LVLEINSLKY AYNVSLKEVM QVLSHVVLEF PLQQMDSPLD SSRYCALLLP LLKAWSPVFR NYIKRAA DH LEALAAIEDF FLEHEALGIS MAKVLMAFYQ LEILAEETIL SWFSQRDTTD KGQQLRKNQQ LQRFIQWLKE AEEESSED D UniProtKB: Translation initiation factor eIF2B subunit epsilon |
-Macromolecule #2: Translation initiation factor eIF-2B subunit beta
| Macromolecule | Name: Translation initiation factor eIF-2B subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.008578 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MHHHHHHGGG SENLYFQSPG SAAKGSELSE RIESFVETLK RGGGPRSSEE MARETLGLLR QIITDHRWSN AGELMELIRR EGRRMTAAQ PSETTVGNMV RRVLKIIREE YGRLHGRSDE SDQQESLHKL LTSGGLNEDF SFHYAQLQSN IIEAINELLV E LEGTMENI ...String: MHHHHHHGGG SENLYFQSPG SAAKGSELSE RIESFVETLK RGGGPRSSEE MARETLGLLR QIITDHRWSN AGELMELIRR EGRRMTAAQ PSETTVGNMV RRVLKIIREE YGRLHGRSDE SDQQESLHKL LTSGGLNEDF SFHYAQLQSN IIEAINELLV E LEGTMENI AAQALEHIHS NEVIMTIGFS RTVEAFLKEA ARKRKFHVIV AECAPFCQGH EMAVNLSKAG IETTVMTDAA IF AVMSRVN KVIIGTKTIL ANGALRAVTG THTLALAAKH HSTPLIVCAP MFKLSPQFPN EEDSFHKFVA PEEVLPFTEG DIL EKVSVH CPVFDYVPPE LITLFISNIG GNAPSYIYRL MSELYHPDDH VL UniProtKB: Translation initiation factor eIF2B subunit beta |
-Macromolecule #3: Translation initiation factor eIF-2B subunit delta
| Macromolecule | Name: Translation initiation factor eIF-2B subunit delta / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 57.640168 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAAVAVAVRE DSGSGMKAEL PPGPGAVGRE MTKEEKLQLR KEKKQQKKKR KEEKGAEPET GSAVSAAQCQ VGPTRELPES GIQLGTPRE KVPAGRSKAE LRAERRAKQE AERALKQARK GEQGGPPPKA SPSTAGETPS GVKRLPEYPQ VDDLLLRRLV K KPERQQVP ...String: MAAVAVAVRE DSGSGMKAEL PPGPGAVGRE MTKEEKLQLR KEKKQQKKKR KEEKGAEPET GSAVSAAQCQ VGPTRELPES GIQLGTPRE KVPAGRSKAE LRAERRAKQE AERALKQARK GEQGGPPPKA SPSTAGETPS GVKRLPEYPQ VDDLLLRRLV K KPERQQVP TRKDYGSKVS LFSHLPQYSR QNSLTQFMSI PSSVIHPAMV RLGLQYSQGL VSGSNARCIA LLRALQQVIQ DY TTPPNEE LSRDLVNKLK PYMSFLTQCR PLSASMHNAI KFLNKEITSV GSSKREEEAK SELRAAIDRY VQEKIVLAAQ AIS RFAYQK ISNGDVILVY GCSSLVSRIL QEAWTEGRRF RVVVVDSRPW LEGRHTLRSL VHAGVPASYL LIPAASYVLP EVSK VLLGA HALLANGSVM SRVGTAQLAL VARAHNVPVL VCCETYKFCE RVQTDAFVSN ELDDPDDLQC KRGEHVALAN WQNHA SLRL LNLVYDVTPP ELVDLVITEL GMIPCSSVPV VLRVKSSDQ UniProtKB: Translation initiation factor eIF2B subunit delta |
-Macromolecule #4: Translation initiation factor eIF-2B subunit alpha
| Macromolecule | Name: Translation initiation factor eIF-2B subunit alpha / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 33.754148 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDDKELIEYF KSQMKEDPDM ASAVAAIRTL LEFLKRDKGE TIQGLRANLT SAIETLCGVD SSVAVSSGGE LFLRFISLAS LEYSDYSKC KKIMIERGEL FLRRISLSRN KIADLCHTFI KDGATILTHA YSRVVLRVLE AAVAAKKRFS VYVTESQPDL S GKKMAKAL ...String: MDDKELIEYF KSQMKEDPDM ASAVAAIRTL LEFLKRDKGE TIQGLRANLT SAIETLCGVD SSVAVSSGGE LFLRFISLAS LEYSDYSKC KKIMIERGEL FLRRISLSRN KIADLCHTFI KDGATILTHA YSRVVLRVLE AAVAAKKRFS VYVTESQPDL S GKKMAKAL CHLNVPVTVV LDAAVGYIME KADLVIVGAE GVVENGGIIN KIGTNQMAVC AKAQNKPFYV VAESFKFVRL FP LNQQDVP DKFKYKADTL KVAQTGQDLK EEHPWVDYTA PSLITLLFTD LGVLTPSAVS DELIKLYL UniProtKB: Translation initiation factor eIF2B subunit alpha |
-Macromolecule #5: Translation initiation factor eIF-2B subunit gamma
| Macromolecule | Name: Translation initiation factor eIF-2B subunit gamma / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 50.30423 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MEFQAVVMAV GGGSRMTDLT SSIPKPLLPV GNKPLIWYPL NLLERVGFEE VIVVTTRDVQ KALCAEFKMK MKPDIVCIPD DADMGTADS LRYIYPKLKT DVLVLSCDLI TDVALHEVVD LFRAYDASLA MLMRKGQDSI EPVPGQKGKK KAVEQRDFIG V DSTGKRLL ...String: MEFQAVVMAV GGGSRMTDLT SSIPKPLLPV GNKPLIWYPL NLLERVGFEE VIVVTTRDVQ KALCAEFKMK MKPDIVCIPD DADMGTADS LRYIYPKLKT DVLVLSCDLI TDVALHEVVD LFRAYDASLA MLMRKGQDSI EPVPGQKGKK KAVEQRDFIG V DSTGKRLL FMANEADLDE ELVIKGSILQ KHPRIRFHTG LVDAHLYCLK KYIVDFLMEN GSITSIRSEL IPYLVRKQFS SA SSQQGQE EKEEDLKKKE LKSLDIYSFI KEANTLNLAP YDACWNACRG DRWEDLSRSQ VRCYVHIMKE GLCSRVSTLG LYM EANRQV PKLLSALCPE EPPVHSSAQI VSKHLVGVDS LIGPETQIGE KSSIKRSVIG SSCLIKDRVT ITNCLLMNSV TVEE GSNIQ GSVICNNAVI EKGADIKDCL IGSGQRIEAK AKRVNEVIVG NDQLMEI UniProtKB: Translation initiation factor eIF2B subunit gamma |
-Macromolecule #6: Eukaryotic translation initiation factor 2 subunit 1
| Macromolecule | Name: Eukaryotic translation initiation factor 2 subunit 1 / type: protein_or_peptide / ID: 6 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.238121 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDYKDDDDKP GLSCRFYQHK FPEVEDVVMV NVRSIAEMGA YVSLLEYNNI EGMILLSEL(SEP) RRRIRSINKL IRIGRN ECV VVIRVDKEKG YIDLSKRRVS PEEAIKCEDK FTKSKTVYSI LRHVAEVLEY TKDEQLESLF QRTAWVFDDK YKRPGYG AY DAFKHAVSDP ...String: MDYKDDDDKP GLSCRFYQHK FPEVEDVVMV NVRSIAEMGA YVSLLEYNNI EGMILLSEL(SEP) RRRIRSINKL IRIGRN ECV VVIRVDKEKG YIDLSKRRVS PEEAIKCEDK FTKSKTVYSI LRHVAEVLEY TKDEQLESLF QRTAWVFDDK YKRPGYG AY DAFKHAVSDP SILDSLDLNE DEREVLINNI NRRLTPQAVK IRADIEVACY GYEGIDAVKE ALRAGLNCST ENMPIKIN L IAPPRYVMTT TTLERTEGLS VLSQAMAVIK EKIEEKRGVF NVQMEPKVVT DTDETELARQ MERLERENAE VDGDDDAEE MEAKAED UniProtKB: Eukaryotic translation initiation factor 2 subunit 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 80.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















