+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0471 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the NKCC1 CTD | |||||||||
 Map data Map data | Cytosolic domain | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationswim bladder inflation / Cation-coupled Chloride cotransporters / chloride:monoatomic cation symporter activity / sodium:potassium:chloride symporter activity / ammonium transmembrane transport / sodium ion homeostasis / ammonium channel activity / chloride ion homeostasis / ear development / potassium ion homeostasis ...swim bladder inflation / Cation-coupled Chloride cotransporters / chloride:monoatomic cation symporter activity / sodium:potassium:chloride symporter activity / ammonium transmembrane transport / sodium ion homeostasis / ammonium channel activity / chloride ion homeostasis / ear development / potassium ion homeostasis / cell volume homeostasis / inner ear morphogenesis / potassium ion import across plasma membrane / sodium ion transmembrane transport / chloride transmembrane transport / basolateral plasma membrane / apical plasma membrane / metal ion binding / identical protein binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
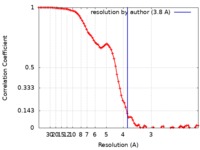
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Feng L / Liao MF | |||||||||
 Citation Citation |  Journal: Nature / Year: 2019 Journal: Nature / Year: 2019Title: Structure and mechanism of the cation-chloride cotransporter NKCC1. Authors: Thomas A Chew / Benjamin J Orlando / Jinru Zhang / Naomi R Latorraca / Amy Wang / Scott A Hollingsworth / Dong-Hua Chen / Ron O Dror / Maofu Liao / Liang Feng /  Abstract: Cation-chloride cotransporters (CCCs) mediate the electroneutral transport of chloride, potassium and/or sodium across the membrane. They have critical roles in regulating cell volume, controlling ...Cation-chloride cotransporters (CCCs) mediate the electroneutral transport of chloride, potassium and/or sodium across the membrane. They have critical roles in regulating cell volume, controlling ion absorption and secretion across epithelia, and maintaining intracellular chloride homeostasis. These transporters are primary targets for some of the most commonly prescribed drugs. Here we determined the cryo-electron microscopy structure of the Na-K-Cl cotransporter NKCC1, an extensively studied member of the CCC family, from Danio rerio. The structure defines the architecture of this protein family and reveals how cytosolic and transmembrane domains are strategically positioned for communication. Structural analyses, functional characterizations and computational studies reveal the ion-translocation pathway, ion-binding sites and key residues for transport activity. These results provide insights into ion selectivity, coupling and translocation, and establish a framework for understanding the physiological functions of CCCs and interpreting disease-related mutations. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0471.map.gz emd_0471.map.gz | 37.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0471-v30.xml emd-0471-v30.xml emd-0471.xml emd-0471.xml | 12.4 KB 12.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0471_fsc.xml emd_0471_fsc.xml | 9.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_0471.png emd_0471.png | 82.6 KB | ||
| Filedesc metadata |  emd-0471.cif.gz emd-0471.cif.gz | 5.6 KB | ||
| Others |  emd_0471_additional.map.gz emd_0471_additional.map.gz | 25.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0471 http://ftp.pdbj.org/pub/emdb/structures/EMD-0471 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0471 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0471 | HTTPS FTP |
-Validation report
| Summary document |  emd_0471_validation.pdf.gz emd_0471_validation.pdf.gz | 605.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_0471_full_validation.pdf.gz emd_0471_full_validation.pdf.gz | 604.9 KB | Display | |
| Data in XML |  emd_0471_validation.xml.gz emd_0471_validation.xml.gz | 9.9 KB | Display | |
| Data in CIF |  emd_0471_validation.cif.gz emd_0471_validation.cif.gz | 12.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0471 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0471 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0471 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0471 | HTTPS FTP |
-Related structure data
| Related structure data |  6npjMC  0470C  0472C  0473C  0474C  0475C  6nphC  6npkC  6nplC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0471.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0471.map.gz / Format: CCP4 / Size: 40.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cytosolic domain | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.055 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: Cytosolic domain
| File | emd_0471_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cytosolic domain | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : cytosolic domain
| Entire | Name: cytosolic domain |
|---|---|
| Components |
|
-Supramolecule #1: cytosolic domain
| Supramolecule | Name: cytosolic domain / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 150 KDa |
-Macromolecule #1: Sodium-potassium-chloride cotransporter 1
| Macromolecule | Name: Sodium-potassium-chloride cotransporter 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 49.444598 KDa |
| Recombinant expression | Organism:  Autographa californica nucleopolyhedrovirus Autographa californica nucleopolyhedrovirus |
| Sequence | String: WGSSTQALTY HQALTHSLQL CGVADHIKTF RPQCLVMTGA PNSRPAILHL VHAFTKNVGL MLCGHVRISS RRPNFKELNS DMLRYQRWL LNNNSKAFYT CVVAEDLRQG TQYMLQAAGL GRLRPNTLVI GFKNDWRIGD IKEVETYINL IHDAFDFQYG V VILRLREG ...String: WGSSTQALTY HQALTHSLQL CGVADHIKTF RPQCLVMTGA PNSRPAILHL VHAFTKNVGL MLCGHVRISS RRPNFKELNS DMLRYQRWL LNNNSKAFYT CVVAEDLRQG TQYMLQAAGL GRLRPNTLVI GFKNDWRIGD IKEVETYINL IHDAFDFQYG V VILRLREG LDISHIQGQD DSSGMKDVVV SVDISKDSDG DSSKPSSKAT SVQNSPAVQK DKKSPTVPLN VADQRLLDSQ QF QQKQGKG TVDVWWLFDD GGLTLLIPYL IANKKKWKDC KIRVFIGGKI NRIDHDRRAM ATLLSKFRID FSDITVLGDI NTK PKSEGL TEFAEMIEPY KLREDDMEQE AAEKLKSEEP WRITANELEL YKAKGNRQIR LNELLKEHSS TANLIVMSMP LARK GAVSS ALYMAWLDTL SKDLPPILLV RGNHQSVLTF YS UniProtKB: Solute carrier family 12 member 2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 7.9 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: Quantifoil R2/1 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 53.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)