+ Open data
Open data
- Basic information
Basic information
| Entry | Database: SASBDB / ID: SASDCR2 |
|---|---|
 Sample Sample | Bromodomain-containing protein 4 (BRD4) tandem bromodomains
|
| Function / homology |  Function and homology information Function and homology informationhistone H4K8ac reader activity / histone H3K9ac reader activity / RNA polymerase II C-terminal domain binding / histone H3K27ac reader activity / P-TEFb complex binding / negative regulation of DNA damage checkpoint / histone H4 reader activity / histone H4K5ac reader activity / histone H4K12ac reader activity / host-mediated suppression of viral transcription ...histone H4K8ac reader activity / histone H3K9ac reader activity / RNA polymerase II C-terminal domain binding / histone H3K27ac reader activity / P-TEFb complex binding / negative regulation of DNA damage checkpoint / histone H4 reader activity / histone H4K5ac reader activity / histone H4K12ac reader activity / host-mediated suppression of viral transcription / histone H4K16ac reader activity / positive regulation of G2/M transition of mitotic cell cycle / positive regulation of T-helper 17 cell lineage commitment / RNA polymerase II CTD heptapeptide repeat kinase activity / condensed nuclear chromosome / transcription coregulator activity / positive regulation of transcription elongation by RNA polymerase II / p53 binding / chromosome / regulation of inflammatory response / histone binding / Potential therapeutics for SARS / transcription coactivator activity / positive regulation of canonical NF-kappaB signal transduction / transcription cis-regulatory region binding / chromatin remodeling / protein serine/threonine kinase activity / DNA damage response / chromatin binding / regulation of transcription by RNA polymerase II / positive regulation of DNA-templated transcription / chromatin / enzyme binding / positive regulation of transcription by RNA polymerase II / nucleoplasm / nucleus Similarity search - Function |
| Biological species |  Homo sapiens (human) Homo sapiens (human) |
 Citation Citation |  Journal: Mol Cell / Year: 2019 Journal: Mol Cell / Year: 2019Title: Interactome Rewiring Following Pharmacological Targeting of BET Bromodomains. Authors: Jean-Philippe Lambert / Sarah Picaud / Takao Fujisawa / Huayun Hou / Pavel Savitsky / Liis Uusküla-Reimand / Gagan D Gupta / Hala Abdouni / Zhen-Yuan Lin / Monika Tucholska / James D R ...Authors: Jean-Philippe Lambert / Sarah Picaud / Takao Fujisawa / Huayun Hou / Pavel Savitsky / Liis Uusküla-Reimand / Gagan D Gupta / Hala Abdouni / Zhen-Yuan Lin / Monika Tucholska / James D R Knight / Beatriz Gonzalez-Badillo / Nicole St-Denis / Joseph A Newman / Manuel Stucki / Laurence Pelletier / Nuno Bandeira / Michael D Wilson / Panagis Filippakopoulos / Anne-Claude Gingras /      Abstract: Targeting bromodomains (BRDs) of the bromo-and-extra-terminal (BET) family offers opportunities for therapeutic intervention in cancer and other diseases. Here, we profile the interactomes of BRD2, ...Targeting bromodomains (BRDs) of the bromo-and-extra-terminal (BET) family offers opportunities for therapeutic intervention in cancer and other diseases. Here, we profile the interactomes of BRD2, BRD3, BRD4, and BRDT following treatment with the pan-BET BRD inhibitor JQ1, revealing broad rewiring of the interaction landscape, with three distinct classes of behavior for the 603 unique interactors identified. A group of proteins associate in a JQ1-sensitive manner with BET BRDs through canonical and new binding modes, while two classes of extra-terminal (ET)-domain binding motifs mediate acetylation-independent interactions. Last, we identify an unexpected increase in several interactions following JQ1 treatment that define negative functions for BRD3 in the regulation of rRNA synthesis and potentially RNAPII-dependent gene expression that result in decreased cell proliferation. Together, our data highlight the contributions of BET protein modules to their interactomes allowing for a better understanding of pharmacological rewiring in response to JQ1. |
 Contact author Contact author |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
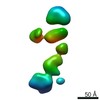
-Models
| Model #1183 |  Type: dummy / Radius of dummy atoms: 3.75 A / Chi-square value: 1.047 / P-value: 0.027000  Search similar-shape structures of this assembly by Omokage search (details) Search similar-shape structures of this assembly by Omokage search (details) |
|---|
- Sample
Sample
 Sample Sample | Name: Bromodomain-containing protein 4 (BRD4) tandem bromodomains Specimen concentration: 13 mg/ml |
|---|---|
| Buffer | Name: 20 mM HEPES, 150 mM NaCl, 2% glycerol, 0.5 mM TCEP / pH: 7.5 |
| Entity #617 | Name: BRD4 / Type: protein / Description: Bromodomain-containing protein 4 / Formula weight: 47.243 / Num. of mol.: 1 / Source: Homo sapiens / References: UniProt: O60885 Sequence: SMNPPPPETS NPNKPKRQTN QLQYLLRVVL KTLWKHQFAW PFQQPVDAVK LNLPDYYKII KTPMDMGTIK KRLENNYYWN AQECIQDFNT MFTNCYIYNK PGDDIVLMAE ALEKLFLQKI NELPTEETEI MIVQAKGRGR GRKETGTAKP GVSTVPNTTQ ASTPPQTQTP ...Sequence: SMNPPPPETS NPNKPKRQTN QLQYLLRVVL KTLWKHQFAW PFQQPVDAVK LNLPDYYKII KTPMDMGTIK KRLENNYYWN AQECIQDFNT MFTNCYIYNK PGDDIVLMAE ALEKLFLQKI NELPTEETEI MIVQAKGRGR GRKETGTAKP GVSTVPNTTQ ASTPPQTQTP QPNPPPVQAT PHPFPAVTPD LIVQTPVMTV VPPQPLQTPP PVPPQPQPPP APAPQPVQSH PPIIAATPQP VKTKKGVKRK ADTTTPTTID PIHEPPSLPP EPKTTKLGQR RESSRPVKPP KKDVPDSQQH PAPEKSSKVS EQLKCCSGIL KEMFAKKHAA YAWPFYKPVD VEALGLHDYC DIIKHPMDMS TIKSKLEARE YRDAQEFGAD VRLMFSNCYK YNPPDHEVVA MARKLQDVFE MRFAKMPDE |
-Experimental information
| Beam | Instrument name: Diamond Light Source B21 / City: Oxfordshire / 国: UK  / Shape: 1 x 5 mm / Type of source: X-ray synchrotron / Wavelength: 0.1 Å / Dist. spec. to detc.: 3.929 mm / Shape: 1 x 5 mm / Type of source: X-ray synchrotron / Wavelength: 0.1 Å / Dist. spec. to detc.: 3.929 mm | |||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Detector | Name: Pilatus 2M | |||||||||||||||||||||||||||||||||||||||
| Scan | Measurement date: Jan 13, 2017 / Storage temperature: 10 °C / Cell temperature: 10 °C / Exposure time: 10 sec. / Number of frames: 12 / Unit: 1/A /
| |||||||||||||||||||||||||||||||||||||||
| Distance distribution function P(R) |
| |||||||||||||||||||||||||||||||||||||||
| Result |
|
 Movie
Movie Controller
Controller


 SASDCR2
SASDCR2