[English] 日本語
 Yorodumi
Yorodumi- PDB-7e7b: Cryo-EM structure of the SARS-CoV-2 furin site mutant S-Trimer fr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7e7b | ||||||
|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the SARS-CoV-2 furin site mutant S-Trimer from a subunit vaccine candidate | ||||||
 Components Components | Spike glycoprotein,Collagen alpha-1(I) chain | ||||||
 Keywords Keywords | VIRAL PROTEIN / spike protein / COVID-19 / vaccine | ||||||
| Function / homology |  Function and homology information Function and homology informationcollagen type I trimer / cellular response to vitamin E / tooth mineralization / cellular response to fluoride / Anchoring fibril formation / Crosslinking of collagen fibrils / collagen biosynthetic process / Collagen chain trimerization / Defective VWF binding to collagen type I / extracellular matrix structural constituent conferring tensile strength ...collagen type I trimer / cellular response to vitamin E / tooth mineralization / cellular response to fluoride / Anchoring fibril formation / Crosslinking of collagen fibrils / collagen biosynthetic process / Collagen chain trimerization / Defective VWF binding to collagen type I / extracellular matrix structural constituent conferring tensile strength / platelet-derived growth factor binding / Enhanced cleavage of VWF variant by ADAMTS13 / Defective VWF cleavage by ADAMTS13 variant / bone trabecula formation / Enhanced binding of GP1BA variant to VWF multimer:collagen / Defective binding of VWF variant to GPIb:IX:V / intramembranous ossification / Extracellular matrix organization / cartilage development involved in endochondral bone morphogenesis / embryonic skeletal system development / Collagen biosynthesis and modifying enzymes / skin morphogenesis / collagen-activated tyrosine kinase receptor signaling pathway / endochondral ossification / Platelet Adhesion to exposed collagen / collagen fibril organization / face morphogenesis / response to steroid hormone / Assembly of collagen fibrils and other multimeric structures / Scavenging by Class A Receptors / MET activates PTK2 signaling / GP1b-IX-V activation signalling / Syndecan interactions / skin development / blood vessel development / RUNX2 regulates osteoblast differentiation / Platelet Aggregation (Plug Formation) / Collagen degradation / Non-integrin membrane-ECM interactions / cellular response to fibroblast growth factor stimulus / protein localization to nucleus / negative regulation of cell-substrate adhesion / ECM proteoglycans / response to hyperoxia / Integrin cell surface interactions / positive regulation of epithelial to mesenchymal transition / response to mechanical stimulus / response to cAMP / cellular response to transforming growth factor beta stimulus / GPVI-mediated activation cascade / cellular response to retinoic acid / extracellular matrix organization / cellular response to epidermal growth factor stimulus / visual perception / ossification / secretory granule / skeletal system development / Cell surface interactions at the vascular wall / response to insulin / cellular response to amino acid stimulus / sensory perception of sound / cellular response to glucose stimulus / response to hydrogen peroxide / cellular response to mechanical stimulus / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / osteoblast differentiation / positive regulation of canonical Wnt signaling pathway / protein transport / cellular response to tumor necrosis factor / response to estradiol / protease binding / : / Maturation of spike protein / viral translation / Translation of Structural Proteins / host cell surface / Virion Assembly and Release / host extracellular space / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell / membrane fusion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / Attachment and Entry / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / positive regulation of cell migration / receptor ligand activity / endocytosis involved in viral entry into host cell / response to xenobiotic stimulus / endoplasmic reticulum lumen / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / positive regulation of DNA-templated transcription / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses Similarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.6 Å | ||||||
 Authors Authors | Zheng, S. / Ma, J. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: J Virol / Year: 2021 Journal: J Virol / Year: 2021Title: Cryo-EM structure of S-Trimer, a subunit vaccine candidate for COVID-19. Authors: Jiahao Ma / Danmei Su / Yinyan Sun / Xueqin Huang / Ying Liang / Linqiang Fang / Yan Ma / Wenhui Li / Peng Liang / Sanduo Zheng /  Abstract: Within a year after its emergence, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 100 million people worldwide with a death toll over 2 million. Vaccination ...Within a year after its emergence, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected over 100 million people worldwide with a death toll over 2 million. Vaccination remains the best hope to ultimately put this pandemic to an end. Here, using Trimer-Tag technology, we produced both wild-type (WT) and furin site mutant (MT) S-Trimers for COVID-19 vaccine studies. Cryo-EM structures of the WT and MT S-Trimers, determined at 3.2 Å and 2.6 Å respectively, revealed that both antigens adopt a tightly closed conformation and their structures are essentially identical to that of the previously solved full-length WT S protein in detergent. The tightly closed conformation is stabilized by fatty acid and polysorbate 80 binding at the receptor binding domains (RBDs) and the N terminal domains (NTDs) respectively. Additionally, we identified an important pH switch in the WT S-Trimer that shows dramatic conformational change and accounts for its increased stability at lower pH. These results validate Trimer-Tag as a platform technology in production of metastable WT S-Trimer as a candidate for COVID-19 subunit vaccine.Effective vaccine against SARS-CoV-2 is critical to end the COVID-19 pandemic. Here, using Trimer-Tag technology, we are able to produce stable and large quantities of WT S-Trimer, a subunit vaccine candidate for COVID-19 with high safety and efficacy from animal and Phase 1 clinical trial studies. Cryo-EM structures of the S-Trimer subunit vaccine candidate show that it predominately adopts tightly closed pre-fusion state, and resembles that of the native and full-length spike in detergent, confirming its structural integrity. WT S-Trimer is currently being evaluated in global Phase 2/3 clinical trial. Combining with published structures of the S protein, we also propose a model to dissect the conformation change of the spike protein before receptor binding. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7e7b.cif.gz 7e7b.cif.gz | 623.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7e7b.ent.gz pdb7e7b.ent.gz | 505.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7e7b.json.gz 7e7b.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  7e7b_validation.pdf.gz 7e7b_validation.pdf.gz | 3.4 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  7e7b_full_validation.pdf.gz 7e7b_full_validation.pdf.gz | 3.5 MB | Display | |
| Data in XML |  7e7b_validation.xml.gz 7e7b_validation.xml.gz | 95.6 KB | Display | |
| Data in CIF |  7e7b_validation.cif.gz 7e7b_validation.cif.gz | 146.9 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e7/7e7b https://data.pdbj.org/pub/pdb/validation_reports/e7/7e7b ftp://data.pdbj.org/pub/pdb/validation_reports/e7/7e7b ftp://data.pdbj.org/pub/pdb/validation_reports/e7/7e7b | HTTPS FTP |
-Related structure data
| Related structure data |  30998MC  7e7dC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 3 molecules ABC
| #1: Protein | Mass: 168078.203 Da / Num. of mol.: 3 / Mutation: R685A Source method: isolated from a genetically manipulated source Details: Chimeric protein Source: (gene. exp.)   Homo sapiens (human) Homo sapiens (human)Gene: S, 2, COL1A1 / Production host:  |
|---|
-Sugars , 2 types, 54 molecules 
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #3: Sugar | ChemComp-NAG / |
|---|
-Non-polymers , 3 types, 42 molecules 
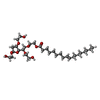



| #4: Chemical | | #5: Chemical | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: SARS-CoV-2 spike protein fused to the C-terminal region of human type 1a collagen Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Experimental value: NO | ||||||||||||
| Source (natural) |
| ||||||||||||
| Source (recombinant) | Organism:  | ||||||||||||
| Buffer solution | pH: 7.4 | ||||||||||||
| Specimen | Conc.: 0.3 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES / Details: monodisperse | ||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 400 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK I / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 282 K Details: blot time 2 seconds, blot force 4, waiting time 8 seconds |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 64000 X / Calibrated defocus min: 600 nm / Calibrated defocus max: 2800 nm / Cs: 0 mm / Alignment procedure: BASIC |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Num. of grids imaged: 2 / Num. of real images: 2000 |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.16_3549: / Classification: refinement | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1504770 | ||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C3 (3 fold cyclic) | ||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 384013 / Symmetry type: POINT | ||||||||||||||||||||||||||||||
| Atomic model building | Space: REAL | ||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 6VXX Accession code: 6VXX / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj



























