[English] 日本語
 Yorodumi
Yorodumi- PDB-7bve: Cryo-EM structure of Mycobacterium smegmatis arabinosyltransferas... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7bve | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Mycobacterium smegmatis arabinosyltransferase EmbC2-AcpM2 in complex with ethambutol | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords | TRANSFERASE / Mycobacterium smegmatis / cell wall synthesis / drug target / ethambutol / arabinosyltransferase / EmbC / acyl carrier protein / arabinogalactan / lipoarabinomannan / drug resistance | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationindolylacetylinositol arabinosyltransferase / indolylacetylinositol arabinosyltransferase activity / arabinosyltransferase activity / Actinobacterium-type cell wall biogenesis / lipid A biosynthetic process / acyl binding / acyl carrier activity / Transferases; Glycosyltransferases; Pentosyltransferases / cell wall organization / metal ion binding ...indolylacetylinositol arabinosyltransferase / indolylacetylinositol arabinosyltransferase activity / arabinosyltransferase activity / Actinobacterium-type cell wall biogenesis / lipid A biosynthetic process / acyl binding / acyl carrier activity / Transferases; Glycosyltransferases; Pentosyltransferases / cell wall organization / metal ion binding / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||||||||
| Biological species |  Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) | |||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.81 Å | |||||||||||||||
 Authors Authors | Zhang, L. / Zhao, Y. / Gao, Y. / Wang, Q. / Li, J. / Besra, G.S. / Rao, Z. | |||||||||||||||
| Funding support |  China, China,  United Kingdom, 4items United Kingdom, 4items
| |||||||||||||||
 Citation Citation |  Journal: Science / Year: 2020 Journal: Science / Year: 2020Title: Structures of cell wall arabinosyltransferases with the anti-tuberculosis drug ethambutol. Authors: Lu Zhang / Yao Zhao / Yan Gao / Lijie Wu / Ruogu Gao / Qi Zhang / Yinan Wang / Chengyao Wu / Fangyu Wu / Sudagar S Gurcha / Natacha Veerapen / Sarah M Batt / Wei Zhao / Ling Qin / Xiuna Yang ...Authors: Lu Zhang / Yao Zhao / Yan Gao / Lijie Wu / Ruogu Gao / Qi Zhang / Yinan Wang / Chengyao Wu / Fangyu Wu / Sudagar S Gurcha / Natacha Veerapen / Sarah M Batt / Wei Zhao / Ling Qin / Xiuna Yang / Manfu Wang / Yan Zhu / Bing Zhang / Lijun Bi / Xian'en Zhang / Haitao Yang / Luke W Guddat / Wenqing Xu / Quan Wang / Jun Li / Gurdyal S Besra / Zihe Rao /    Abstract: The arabinosyltransferases EmbA, EmbB, and EmbC are involved in cell wall synthesis and are recognized as targets for the anti-tuberculosis drug ethambutol. In this study, we determined cryo- ...The arabinosyltransferases EmbA, EmbB, and EmbC are involved in cell wall synthesis and are recognized as targets for the anti-tuberculosis drug ethambutol. In this study, we determined cryo-electron microscopy and x-ray crystal structures of mycobacterial EmbA-EmbB and EmbC-EmbC complexes in the presence of their glycosyl donor and acceptor substrates and with ethambutol. These structures show how the donor and acceptor substrates bind in the active site and how ethambutol inhibits arabinosyltransferases by binding to the same site as both substrates in EmbB and EmbC. Most drug-resistant mutations are located near the ethambutol binding site. Collectively, our work provides a structural basis for understanding the biochemical function and inhibition of arabinosyltransferases and the development of new anti-tuberculosis agents. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7bve.cif.gz 7bve.cif.gz | 519.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7bve.ent.gz pdb7bve.ent.gz | 398.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7bve.json.gz 7bve.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bv/7bve https://data.pdbj.org/pub/pdb/validation_reports/bv/7bve ftp://data.pdbj.org/pub/pdb/validation_reports/bv/7bve ftp://data.pdbj.org/pub/pdb/validation_reports/bv/7bve | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  30217MC  7bvcC  7bvfC  7bvgC  7bvhC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 2 types, 4 molecules ABCD
| #1: Protein | Mass: 116878.898 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria) Mycolicibacterium smegmatis (strain ATCC 700084 / mc(2)155) (bacteria)Strain: ATCC 700084 / mc(2)155 / Gene: embC, MSMEI_6219 Production host:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria)References: UniProt: I7FMU5, UniProt: A0R612*PLUS, indolylacetylinositol arabinosyltransferase #2: Protein | Mass: 10743.876 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria)References: UniProt: A0R0B3 |
|---|
-Non-polymers , 4 types, 8 molecules 

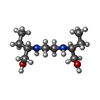




| #3: Chemical | | #4: Chemical | #5: Chemical | #6: Chemical | |
|---|
-Details
| Has ligand of interest | Y |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source (natural) |
| ||||||||||||||||||||||||
| Source (recombinant) | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) | ||||||||||||||||||||||||
| Buffer solution | pH: 7.4 | ||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||
| Specimen | Conc.: 5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES / Details: The sample was monodisperse. | ||||||||||||||||||||||||
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 29000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm / Calibrated defocus min: 1000 nm / Calibrated defocus max: 2000 nm / Cs: 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Temperature (max): 78.6 K / Temperature (min): 78.5 K / Residual tilt: 10 mradians |
| Image recording | Average exposure time: 2 sec. / Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 2 / Num. of real images: 5100 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Image processing | Details: The selected images were normalized. | ||||||||||||||||||||||||||||||||||||
| CTF correction | Details: The CTF correction was done by patch CTF correction. Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1855947 | ||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.81 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 217550 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 62.01 / Protocol: RIGID BODY FIT / Target criteria: correlation coefficient |
 Movie
Movie Controller
Controller













 PDBj
PDBj




