[English] 日本語
 Yorodumi
Yorodumi- PDB-4uy8: Molecular basis for the ribosome functioning as a L-tryptophan se... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4uy8 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Molecular basis for the ribosome functioning as a L-tryptophan sensor - Cryo-EM structure of a TnaC stalled E.coli ribosome | ||||||
 Components Components |
| ||||||
 Keywords Keywords | RIBOSOME / TNAC / TRANSLATION REGULATION | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of L-tryptophan metabolic process / transcriptional attenuation by ribosome / L-tryptophan catabolic process / stringent response / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / translational termination / negative regulation of cytoplasmic translation ...positive regulation of L-tryptophan metabolic process / transcriptional attenuation by ribosome / L-tryptophan catabolic process / stringent response / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / translational termination / negative regulation of cytoplasmic translation / DnaA-L2 complex / translation repressor activity / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / ribosome assembly / regulation of cell growth / DNA-templated transcription termination / response to radiation / mRNA 5'-UTR binding / large ribosomal subunit / ribosome biogenesis / transferase activity / ribosome binding / 5S rRNA binding / ribosomal large subunit assembly / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / ribonucleoprotein complex / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / protein homodimerization activity / DNA binding / RNA binding / zinc ion binding / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.8 Å | ||||||
 Authors Authors | Bischoff, L. / Berninghausen, O. / Beckmann, R. | ||||||
 Citation Citation |  Journal: Cell Rep / Year: 2014 Journal: Cell Rep / Year: 2014Title: Molecular basis for the ribosome functioning as an L-tryptophan sensor. Authors: Lukas Bischoff / Otto Berninghausen / Roland Beckmann /  Abstract: Elevated levels of the free amino acid L-tryptophan (L-Trp) trigger expression of the tryptophanase tnaCAB operon in E. coli. Activation depends on tryptophan-dependent ribosomal stalling during ...Elevated levels of the free amino acid L-tryptophan (L-Trp) trigger expression of the tryptophanase tnaCAB operon in E. coli. Activation depends on tryptophan-dependent ribosomal stalling during translation of the upstream TnaC peptide. Here, we present a cryoelectron microscopy (cryo-EM) reconstruction at 3.8 Å resolution of a ribosome stalled by the TnaC peptide. Unexpectedly, we observe two L-Trp molecules in the ribosomal exit tunnel coordinated within composite hydrophobic pockets formed by the nascent TnaC peptide and the tunnel wall. As a result, the peptidyl transferase center (PTC) adopts a distinct conformation that precludes productive accommodation of release factor 2 (RF2), thereby inducing translational stalling. Collectively, our results demonstrate how the translating ribosome can act as a small molecule sensor for gene regulation. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4uy8.cif.gz 4uy8.cif.gz | 2.3 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4uy8.ent.gz pdb4uy8.ent.gz | 1.8 MB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4uy8.json.gz 4uy8.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uy/4uy8 https://data.pdbj.org/pub/pdb/validation_reports/uy/4uy8 ftp://data.pdbj.org/pub/pdb/validation_reports/uy/4uy8 ftp://data.pdbj.org/pub/pdb/validation_reports/uy/4uy8 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2773MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
+50S RIBOSOMAL PROTEIN ... , 29 types, 29 molecules 0123458CDEFGIJKLMNOPQRSTUWXYZ
-Protein/peptide , 3 types, 3 molecules 67H
| #7: Protein/peptide | Mass: 3300.856 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #8: Protein/peptide | Mass: 2427.801 Da / Num. of mol.: 1 / Fragment: RESIDUES 5-24 / Source method: isolated from a natural source / Source: (natural)  |
| #17: Protein/peptide | Mass: 5467.367 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-RNA chain , 3 types, 3 molecules ABV
| #10: RNA chain | Mass: 925492.125 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #11: RNA chain | Mass: 38177.762 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #31: RNA chain | Mass: 24890.803 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-Non-polymers , 4 types, 585 molecules 

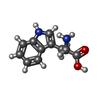




| #36: Chemical | ChemComp-MG / #37: Chemical | ChemComp-ZN / | #38: Chemical | #39: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: TNAC STALLED E.COLI RIBOSOME / Type: RIBOSOME |
|---|---|
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Details: HOLEY CARBON |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE Details: VITRIFICATION 1 --CRYOGEN- ETHANE,INSTRUMENT- FEI VITROBOT MARK IV |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS / Date: Jan 27, 2014 |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) |
- Processing
Processing
| EM software | Name: SPIDER / Category: 3D reconstruction | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Details: MICROGRAPH | ||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||
| 3D reconstruction | Resolution: 3.8 Å / Num. of particles: 72468 Details: SUBMISSION BASED ON EXPERIMENTAL DATA FROM EMDB EMD-2773. (DEPOSITION ID: 12802). Symmetry type: POINT | ||||||||||||
| Refinement | Highest resolution: 3.8 Å | ||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 3.8 Å
|
 Movie
Movie Controller
Controller













 PDBj
PDBj

































