+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6865 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the mechanosensitive Piezo1 channel | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Piezo1 / Piezo2 / mechanogating / channel / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationmechanosensitive monoatomic cation channel activity / cuticular plate / positive regulation of cell-cell adhesion mediated by integrin / positive regulation of integrin activation / detection of mechanical stimulus / mechanosensitive monoatomic ion channel activity / stereocilium / positive regulation of myotube differentiation / lamellipodium membrane / monoatomic cation transport ...mechanosensitive monoatomic cation channel activity / cuticular plate / positive regulation of cell-cell adhesion mediated by integrin / positive regulation of integrin activation / detection of mechanical stimulus / mechanosensitive monoatomic ion channel activity / stereocilium / positive regulation of myotube differentiation / lamellipodium membrane / monoatomic cation transport / monoatomic cation channel activity / endoplasmic reticulum-Golgi intermediate compartment membrane / regulation of membrane potential / endoplasmic reticulum membrane / endoplasmic reticulum / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
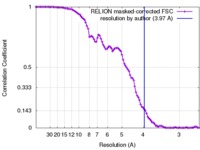
| Method | single particle reconstruction / cryo EM / Resolution: 3.97 Å | |||||||||
 Authors Authors | Zhao Q / Zhou H | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2018 Journal: Nature / Year: 2018Title: Structure and mechanogating mechanism of the Piezo1 channel. Authors: Qiancheng Zhao / Heng Zhou / Shaopeng Chi / Yanfeng Wang / Jianhua Wang / Jie Geng / Kun Wu / Wenhao Liu / Tingxin Zhang / Meng-Qiu Dong / Jiawei Wang / Xueming Li / Bailong Xiao /  Abstract: The mechanosensitive Piezo channels function as key eukaryotic mechanotransducers. However, their structures and mechanogating mechanisms remain unknown. Here we determine the three-bladed, propeller- ...The mechanosensitive Piezo channels function as key eukaryotic mechanotransducers. However, their structures and mechanogating mechanisms remain unknown. Here we determine the three-bladed, propeller-like electron cryo-microscopy structure of mouse Piezo1 and functionally reveal its mechanotransduction components. Despite the lack of sequence repetition, we identify nine repetitive units consisting of four transmembrane helices each-which we term transmembrane helical units (THUs)-which assemble into a highly curved blade-like structure. The last transmembrane helix encloses a hydrophobic pore, followed by three intracellular fenestration sites and side portals that contain pore-property-determining residues. The central region forms a 90 Å-long intracellular beam-like structure, which undergoes a lever-like motion to connect THUs to the pore via the interfaces of the C-terminal domain, the anchor-resembling domain and the outer helix. Deleting extracellular loops in the distal THUs or mutating single residues in the beam impairs the mechanical activation of Piezo1. Overall, Piezo1 possesses a unique 38-transmembrane-helix topology and designated mechanotransduction components, which enable a lever-like mechanogating mechanism. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6865.map.gz emd_6865.map.gz | 9.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6865-v30.xml emd-6865-v30.xml emd-6865.xml emd-6865.xml | 14.7 KB 14.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_6865_fsc.xml emd_6865_fsc.xml | 9.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_6865.png emd_6865.png | 258.7 KB | ||
| Filedesc metadata |  emd-6865.cif.gz emd-6865.cif.gz | 7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6865 http://ftp.pdbj.org/pub/emdb/structures/EMD-6865 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6865 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6865 | HTTPS FTP |
-Related structure data
| Related structure data |  5z10MC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6865.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6865.map.gz / Format: CCP4 / Size: 83.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Cryo-EM map of the mechanosensitive Piezo1 channel
| Entire | Name: Cryo-EM map of the mechanosensitive Piezo1 channel |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM map of the mechanosensitive Piezo1 channel
| Supramolecule | Name: Cryo-EM map of the mechanosensitive Piezo1 channel / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Piezo-type mechanosensitive ion channel component 1
| Macromolecule | Name: Piezo-type mechanosensitive ion channel component 1 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 292.320656 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MEPHVLGAGL YWLLLPCTLL AASLLRFNAL SLVYLLFLLL LPWLPGPSRH SIPGHTGRLL RALLCLSLLF LVAHLAFQIC LHTVPHLDQ FLGQNGSLWV KVSQHIGVTR LDLKDIFNTT RLVAPDLGVL LASSLCLGLC GRLTRKAGQS RRTQELQDDD D DDDDDDED ...String: MEPHVLGAGL YWLLLPCTLL AASLLRFNAL SLVYLLFLLL LPWLPGPSRH SIPGHTGRLL RALLCLSLLF LVAHLAFQIC LHTVPHLDQ FLGQNGSLWV KVSQHIGVTR LDLKDIFNTT RLVAPDLGVL LASSLCLGLC GRLTRKAGQS RRTQELQDDD D DDDDDDED IDAAPAVGLK GAPALATKRR LWLASRFRVT AHWLLMTSGR TLVIVLLALA GIAHPSAFSS IYLVVFLAIC TW WSCHFPL SPLGFNTLCV MVSCFGAGHL ICLYCYQTPF IQDMLPPGNI WARLFGLKNF VDLPNYSSPN ALVLNTKHAW PIY VSPGIL LLLYYTATSL LKLHKSCPSE LRKETPREDE EHELELDHLE PEPQARDATQ GEMPMTTEPD LDNCTVHVLT SQSP VRQRP VRPRLAELKE MSPLHGLGHL IMDQSYVCAL IAMMVWSIMY HSWLTFVLLL WACLIWTVRS RHQLAMLCSP CILLY GLTL CCLRYVWAME LPELPTTLGP VSLHQLGLEH TRYPCLDLGA MLLYLLTFWL LLRQFVKEKL LKKQKVPAAL LEVTVA DTE PTQTQTLLRS LGELVTGIYV KYWIYVCAGM FIVVSFAGRL VVYKIVYMFL FLLCLTLFQV YYTLWRKLLR VFWWLVV AY TMLVLIAVYT FQFQDFPTYW RNLTGFTDEQ LGDLGLEQFS VSELFSSILI PGFFLLACIL QLHYFHRPFM QLTDLEHV P PPGTRHPRWA HRQDAVSEAP LLEHQEEEEV FREDGQSMDG PHQATQVPEG TASKWGLVAD RLLDLAASFS AVLTRIQVF VRRLLELHVF KLVALYTVWV ALKEVSVMNL LLVVLWAFAL PYPRFRPMAS CLSTVWTCII IVCKMLYQLK IVNPHEYSSN CTEPFPNNT NLQPLEINQS LLYRGPVDPA NWFGVRKGYP NLGYIQNHLQ ILLLLVFEAV VYRRQEHYRR QHQQAPLPAQ A VCADGTRQ RLDQDLLSCL KYFINFFFYK FGLEICFLMA VNVIGQRMNF MVILHGCWLV AILTRRRREA IARLWPNYCL FL TLFLLYQ YLLCLGMPPA LCIDYPWRWS KAIPMNSALI KWLYLPDFFR APNSTNLISD FLLLLCASQQ WQVFSAERTE EWQ RMAGIN TDHLEPLRGE PNPIPNFIHC RSYLDMLKVA VFRYLFWLVL VVVFVAGATR ISIFGLGYLL ACFYLLLFGT TLLQ KDTRA QLVLWDCLIL YNVTVIISKN MLSLLSCVFV EQMQSNFCWV IQLFSLVCTV KGYYDPKEMM TRDRDCLLPV EEAGI IWDS ICFFFLLLQR RIFLSHYFLH VSADLKATAL QASRGFALYN AANLKSINFH RQIEEKSLAQ LKRQMKRIRA KQEKYR QSQ ASRGQLQSKD PQDPSQEPGP DSPGGSSPPR RQWWRPWLDH ATVIHSGDYF LFESDSEEEE EALPEDPRPA AQSAFQM AY QAWVTNAQTV LRQRRERARQ ERAEQLASGG DLNPDVEPVD VPEDEMAGRS HMMQRVLSTM QFLWVLGQAT VDGLTRWL R AFTKHHRTMS DVLCAERYLL TQELLRVGEV RRGVLDQLYV GEDEATLSGP VETRDGPSTA SSGLGAEEPL SSMTDDTSS PLSTGYNTRS GSEEIVTDAG DLQAGTSLHG SQELLANART RMRTASELLL DRRLHIPELE EAERFEAQQG RTLRLLRAGY QCVAAHSEL LCYFIIILNH MVTASAASLV LPVLVFLWAM LTIPRPSKRF WMTAIVFTEV MVVTKYLFQF GFFPWNSYVV L RRYENKPY FPPRILGLEK TDSYIKYDLV QLMALFFHRS QLLCYGLWDH EEDRYPKDHC RSSVKDREAK EEPEAKLESQ SE TGTGHPK EPVLAGTPRD HIQGKGSIRS KDVIQDPPED LKPRHTRHIS IRFRRRKETP GPKGTAVMET EHEEGEGKET TER KRPRHT QEKSKFRERM KAAGRRLQSF CVSLAQSFYQ PLQRFFHDIL HTKYRAATDV YALMFLADIV DIIIIIFGFW AFGK HSAAT DIASSLSDDQ VPQAFLFMLL VQFGTMVIDR ALYLRKTVLG KLAFQVVLVV AIHIWMFFIL PAVTERMFSQ NAVAQ LWYF VKCIYFALSA YQIRCGYPTR ILGNFLTKKY NHLNLFLFQG FRLVPFLVEL RAVMDWVWTD TTLSLSNWMC VEDIYA NIF IIKCSRETEK KYPQPKGQKK KKIVKYGMGG LIILFLIAII WFPLLFMSLI RSVVGVVNQP IDVTVTLKLG GYEPLFT MS AQQPSIVPFT PQAYEELSQQ FDPYPLAMQF ISQYSPEDIV TAQIEGSSGA LWRISPPSRA QMKQELYNGT ADITLRFT W NFQRDLAKGG TVEYTNEKHT LELAPNSTAR RQLAQLLEGR PDQSVVIPHL FPKYIRAPNG PEANPVKQLQ PDEEEDYLG VRIQLRREQV GTGASGEQAG TKASDFLEWW VIELQDCKAD CNLLPMVIFS DKVSPPSLGF LAGYGIVGLY VSIVLVVGKF VRGFFSEIS HSIMFEELPC VDRILKLCQD IFLVRETREL ELEEELYAKL IFLYRSPETM IKWTRERE UniProtKB: Piezo-type mechanosensitive ion channel component 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.18 mg/mL |
|---|---|
| Buffer | pH: 7.2 |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 281 K / Instrument: FEI VITROBOT MARK IV Details: After a 15 sec waiting time, the grids were blotted for 3.5 sec and plunged into liquid ethane. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-32 / Average exposure time: 8.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated defocus min: 3.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus min: 1.5 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)