+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6732 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | SARS-CoV spike glycoprotein | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | SARS-CoV / Receptor binding / Membrane fusion / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationMaturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / Attachment and Entry / endocytosis involved in viral entry into host cell / SARS-CoV-1 activates/modulates innate immune responses / suppression by virus of host tetherin activity / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / membrane fusion / positive regulation of viral entry into host cell ...Maturation of spike protein / Translation of Structural Proteins / Virion Assembly and Release / Attachment and Entry / endocytosis involved in viral entry into host cell / SARS-CoV-1 activates/modulates innate immune responses / suppression by virus of host tetherin activity / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / membrane fusion / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / host cell plasma membrane / virion membrane / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  SARS coronavirus / SARS coronavirus /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Gui M / Song W | |||||||||
 Citation Citation |  Journal: Cell Res / Year: 2017 Journal: Cell Res / Year: 2017Title: Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Authors: Miao Gui / Wenfei Song / Haixia Zhou / Jingwei Xu / Silian Chen / Ye Xiang / Xinquan Wang /  Abstract: The global outbreak of SARS in 2002-2003 was caused by the infection of a new human coronavirus SARS-CoV. The infection of SARS-CoV is mediated mainly through the viral surface glycoproteins, which ...The global outbreak of SARS in 2002-2003 was caused by the infection of a new human coronavirus SARS-CoV. The infection of SARS-CoV is mediated mainly through the viral surface glycoproteins, which consist of S1 and S2 subunits and form trimer spikes on the envelope of the virions. Here we report the ectodomain structures of the SARS-CoV surface spike trimer in different conformational states determined by single-particle cryo-electron microscopy. The conformation 1 determined at 4.3 Å resolution is three-fold symmetric and has all the three receptor-binding C-terminal domain 1 (CTD1s) of the S1 subunits in "down" positions. The binding of the "down" CTD1s to the SARS-CoV receptor ACE2 is not possible due to steric clashes, suggesting that the conformation 1 represents a receptor-binding inactive state. Conformations 2-4 determined at 7.3, 5.7 and 6.8 Å resolutions are all asymmetric, in which one RBD rotates away from the "down" position by different angles to an "up" position. The "up" CTD1 exposes the receptor-binding site for ACE2 engagement, suggesting that the conformations 2-4 represent a receptor-binding active state. This conformational change is also required for the binding of SARS-CoV neutralizing antibodies targeting the CTD1. This phenomenon could be extended to other betacoronaviruses utilizing CTD1 of the S1 subunit for receptor binding, which provides new insights into the intermediate states of coronavirus pre-fusion spike trimer during infection. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6732.map.gz emd_6732.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6732-v30.xml emd-6732-v30.xml emd-6732.xml emd-6732.xml | 10.3 KB 10.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_6732_1.png emd_6732_1.png emd_6732_2.png emd_6732_2.png | 215.6 KB 202.5 KB | ||
| Filedesc metadata |  emd-6732.cif.gz emd-6732.cif.gz | 5.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6732 http://ftp.pdbj.org/pub/emdb/structures/EMD-6732 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6732 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6732 | HTTPS FTP |
-Validation report
| Summary document |  emd_6732_validation.pdf.gz emd_6732_validation.pdf.gz | 351 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_6732_full_validation.pdf.gz emd_6732_full_validation.pdf.gz | 350.5 KB | Display | |
| Data in XML |  emd_6732_validation.xml.gz emd_6732_validation.xml.gz | 6.2 KB | Display | |
| Data in CIF |  emd_6732_validation.cif.gz emd_6732_validation.cif.gz | 7.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6732 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6732 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6732 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6732 | HTTPS FTP |
-Related structure data
| Related structure data |  5xlrMC  6679C  6680C  6681C  6682C  5wrgC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6732.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6732.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
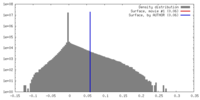
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : SARS-CoV spike glycoprotein
| Entire | Name: SARS-CoV spike glycoprotein |
|---|---|
| Components |
|
-Supramolecule #1: SARS-CoV spike glycoprotein
| Supramolecule | Name: SARS-CoV spike glycoprotein / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  SARS coronavirus SARS coronavirus |
| Molecular weight | Theoretical: 400 KDa |
-Macromolecule #1: Spike glycoprotein
| Macromolecule | Name: Spike glycoprotein / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 133.677312 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MFIFLLFLTL TSGSDLDRCT TFDDVQAPNY TQHTSSMRGV YYPDEIFRSD TLYLTQDLFL PFYSNVTGFH TINHTFGNPV IPFKDGIYF AATEKSNVVR GWVFGSTMNN KSQSVIIINN STNVVIRACN FELCDNPFFA VSKPMGTQTH TMIFDNAFNC T FEYISDAF ...String: MFIFLLFLTL TSGSDLDRCT TFDDVQAPNY TQHTSSMRGV YYPDEIFRSD TLYLTQDLFL PFYSNVTGFH TINHTFGNPV IPFKDGIYF AATEKSNVVR GWVFGSTMNN KSQSVIIINN STNVVIRACN FELCDNPFFA VSKPMGTQTH TMIFDNAFNC T FEYISDAF SLDVSEKSGN FKHLREFVFK NKDGFLYVYK GYQPIDVVRD LPSGFNTLKP IFKLPLGINI TNFRAILTAF SP AQDIWGT SAAAYFVGYL KPTTFMLKYD ENGTITDAVD CSQNPLAELK CSVKSFEIDK GIYQTSNFRV VPSGDVVRFP NIT NLCPFG EVFNATKFPS VYAWERKKIS NCVADYSVLY NSTFFSTFKC YGVSATKLND LCFSNVYADS FVVKGDDVRQ IAPG QTGVI ADYNYKLPDD FMGCVLAWNT RNIDATSTGN YNYKYRYLRH GKLRPFERDI SNVPFSPDGK PCTPPALNCY WPLND YGFY TTTGIGYQPY RVVVLSFELL NAPATVCGPK LSTDLIKNQC VNFNFNGLTG TGVLTPSSKR FQPFQQFGRD VSDFTD SVR DPKTSEILDI SPCSFGGVSV ITPGTNASSE VAVLYQDVNC TDVSTAIHAD QLTPAWRIYS TGNNVFQTQA GCLIGAE HV DTSYECDIPI GAGICASYHT VSLLASTSQK SIVAYTMSLG ADSSIAYSNN TIAIPTNFSI SITTEVMPVS MAKTSVDC N MYICGDSTEC ANLLLQYGSF CTQLNRALSG IAAEQDRNTR EVFAQVKQMY KTPTLKYFGG FNFSQILPDP LKPTKRSFI EDLLFNKVTL ADAGFMKQYG ECLGDINARD LICAQKFNGL TVLPPLLTDD MIAAYTAALV SGTATAGWTF GAGAALQIPF AMQMAYRFN GIGVTQNVLY ENQKQIANQF NKAISQIQES LTTTSTALGK LQDVVNQNAQ ALNTLVKQLS SNFGAISSVL N DILSRLDK VEAEVQIDRL ITGRLQSLQT YVTQQLIRAA EIRASANLAA TKMSECVLGQ SKRVDFCGKG YHLMSFPQAA PH GVVFLHV TYVPSQERNF TTAPAICHEG KAYFPREGVF VFNGTSWFIT QRNFFSPQII TTDNTFVSGN CDVVIGIINN TVY DPLQPE LDSFKEELDK YFKNHTSPDV DLGDISGINA SVVNIQKEID RLNEVAKNLN ESLIDLQELG KYEQYIKWPW SHPQ FEK UniProtKB: Spike glycoprotein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.2 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average exposure time: 8.0 sec. / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C3 (3 fold cyclic) / Resolution.type: BY AUTHOR / Resolution: 3.8 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 1.4) / Number images used: 42135 |
| Initial angle assignment | Type: COMMON LINE |
| Final angle assignment | Type: PROJECTION MATCHING |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)