+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of Eaf3 CHD bound to H3K36me3 nucleosome | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Dynamic Histone Modifications / Gene Regulation / Histone Deacetylase Complex | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnucleosome disassembly/reassembly complex / negative regulation of antisense RNA transcription / Rpd3S complex / regulation of RNA stability / DNA replication-dependent chromatin assembly / regulation of DNA-templated DNA replication initiation / nucleosome disassembly / NuA4 histone acetyltransferase complex / histone acetyltransferase complex / methylated histone binding ...nucleosome disassembly/reassembly complex / negative regulation of antisense RNA transcription / Rpd3S complex / regulation of RNA stability / DNA replication-dependent chromatin assembly / regulation of DNA-templated DNA replication initiation / nucleosome disassembly / NuA4 histone acetyltransferase complex / histone acetyltransferase complex / methylated histone binding / positive regulation of transcription elongation by RNA polymerase II / transcription elongation by RNA polymerase II / structural constituent of chromatin / nucleosome / nucleosome assembly / protein heterodimerization activity / DNA repair / negative regulation of DNA-templated transcription / DNA-templated transcription / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II / DNA binding / nucleus Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
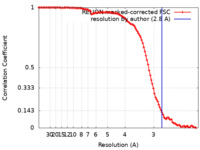
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||||||||
 Authors Authors | Li HT / Yan CY / Guan HP / Wang P | |||||||||||||||
| Funding support |  China, 4 items China, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Diverse modes of H3K36me3-guided nucleosomal deacetylation by Rpd3S. Authors: Haipeng Guan / Pei Wang / Pei Zhang / Chun Ruan / Yutian Ou / Bo Peng / Xiangdong Zheng / Jianlin Lei / Bing Li / Chuangye Yan / Haitao Li /  Abstract: Context-dependent dynamic histone modifications constitute a key epigenetic mechanism in gene regulation. The Rpd3 small (Rpd3S) complex recognizes histone H3 trimethylation on lysine 36 (H3K36me3) ...Context-dependent dynamic histone modifications constitute a key epigenetic mechanism in gene regulation. The Rpd3 small (Rpd3S) complex recognizes histone H3 trimethylation on lysine 36 (H3K36me3) and deacetylates histones H3 and H4 at multiple sites across transcribed regions. Here we solved the cryo-electron microscopy structures of Saccharomyces cerevisiae Rpd3S in its free and H3K36me3 nucleosome-bound states. We demonstrated a unique architecture of Rpd3S, in which two copies of Eaf3-Rco1 heterodimers are asymmetrically assembled with Rpd3 and Sin3 to form a catalytic core complex. Multivalent recognition of two H3K36me3 marks, nucleosomal DNA and linker DNAs by Eaf3, Sin3 and Rco1 positions the catalytic centre of Rpd3 next to the histone H4 N-terminal tail for deacetylation. In an alternative catalytic mode, combinatorial readout of unmethylated histone H3 lysine 4 and H3K36me3 by Rco1 and Eaf3 directs histone H3-specific deacetylation except for the registered histone H3 acetylated lysine 9. Collectively, our work illustrates dynamic and diverse modes of multivalent nucleosomal engagement and methylation-guided deacetylation by Rpd3S, highlighting the exquisite complexity of epigenetic regulation with delicately designed multi-subunit enzymatic machineries in transcription and beyond. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33848.map.gz emd_33848.map.gz | 7.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33848-v30.xml emd-33848-v30.xml emd-33848.xml emd-33848.xml | 24.5 KB 24.5 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_33848_fsc.xml emd_33848_fsc.xml | 10.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_33848.png emd_33848.png | 176 KB | ||
| Others |  emd_33848_half_map_1.map.gz emd_33848_half_map_1.map.gz emd_33848_half_map_2.map.gz emd_33848_half_map_2.map.gz | 80.8 MB 80.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33848 http://ftp.pdbj.org/pub/emdb/structures/EMD-33848 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33848 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33848 | HTTPS FTP |
-Validation report
| Summary document |  emd_33848_validation.pdf.gz emd_33848_validation.pdf.gz | 675.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33848_full_validation.pdf.gz emd_33848_full_validation.pdf.gz | 675.2 KB | Display | |
| Data in XML |  emd_33848_validation.xml.gz emd_33848_validation.xml.gz | 17.5 KB | Display | |
| Data in CIF |  emd_33848_validation.cif.gz emd_33848_validation.cif.gz | 23 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33848 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33848 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33848 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33848 | HTTPS FTP |
-Related structure data
| Related structure data |  7yi1MC  7yi0C  7yi2C  7yi3C  7yi4C  7yi5C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33848.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33848.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0825 Å | ||||||||||||||||||||||||||||||||||||
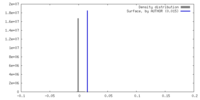
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_33848_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
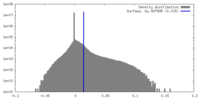
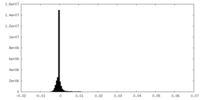
| Density Histograms |
-Half map: #2
| File | emd_33848_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
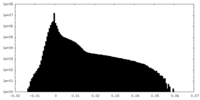
| Density Histograms |
- Sample components
Sample components
+Entire : Rpd3S complex with H3K36me3 nucleosome
+Supramolecule #1: Rpd3S complex with H3K36me3 nucleosome
+Supramolecule #2: Histone H4/H2A/H2B 1.1
+Supramolecule #3: DNA
+Supramolecule #4: EAF3
+Supramolecule #5: Histone H3
+Macromolecule #1: Histone H4
+Macromolecule #2: Histone H2A
+Macromolecule #3: Histone H2B 1.1
+Macromolecule #6: Chromatin modification-related protein EAF3
+Macromolecule #7: Histone H3
+Macromolecule #4: Wisdom 601 DNA (167-MER)
+Macromolecule #5: Wisdom 601 DNA (167-MER)
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 1.8 µm / Calibrated defocus min: 1.5 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)