[English] 日本語
 Yorodumi
Yorodumi- EMDB-31181: Cryo-EM structure of Arabidopsis DCL1 in complex with pri-miRNA 166f -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31181 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Arabidopsis DCL1 in complex with pri-miRNA 166f | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | MicroRNA / miRNA / Endonuclease / Helicase / Hydrolase / Nuclease / RNA-binding | |||||||||
| Function / homology |  Function and homology information Function and homology informationta-siRNA processing / ribonuclease III activity / Hydrolases; Acting on ester bonds; Endoribonucleases producing 5'-phosphomonoesters / helicase activity / rRNA processing / double-stranded RNA binding / DNA binding / ATP binding / metal ion binding / nucleus Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.6 Å | |||||||||
 Authors Authors | Wei X / Ke H | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Plants / Year: 2021 Journal: Nat Plants / Year: 2021Title: Structural basis of microRNA processing by Dicer-like 1. Authors: Xiaobin Wei / Huanhuan Ke / Aijia Wen / Bo Gao / Jing Shi / Yu Feng /  Abstract: MicroRNAs (miRNAs) are short non-coding RNAs that inhibit the expression of target genes by directly binding to their mRNAs. In animals, pri-miRNAs are cleaved by Drosha to generate pre-miRNAs, which ...MicroRNAs (miRNAs) are short non-coding RNAs that inhibit the expression of target genes by directly binding to their mRNAs. In animals, pri-miRNAs are cleaved by Drosha to generate pre-miRNAs, which are subsequently cleaved by Dicer to generate mature miRNAs. Instead of being cleaved by two different enzymes, both cleavages in plants are performed by Dicer-like 1 (DCL1). With a similar domain architecture as human Dicer, it is mysterious how DCL1 recognizes pri-miRNAs and performs two cleavages sequentially. Here, we report the single-particle cryo-electron microscopy structures of Arabidopsis DCL1 complexed with a pri-miRNA and a pre-miRNA, respectively, in cleavage-competent states. These structures uncover the plasticity of the PAZ domain, which is critical for the recognition of both pri-miRNA and pre-miRNA. These structures suggest that the helicase module serves as an engine that transfers the substrate between two sequential cleavage events. This study lays a foundation for dissecting the regulation mechanism of miRNA biogenesis in plants and provides insights into the dicing state of human Dicer. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31181.map.gz emd_31181.map.gz | 22.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31181-v30.xml emd-31181-v30.xml emd-31181.xml emd-31181.xml | 11.3 KB 11.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31181.png emd_31181.png | 44.1 KB | ||
| Filedesc metadata |  emd-31181.cif.gz emd-31181.cif.gz | 6.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31181 http://ftp.pdbj.org/pub/emdb/structures/EMD-31181 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31181 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31181 | HTTPS FTP |
-Related structure data
| Related structure data |  7eldMC  7eleC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31181.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31181.map.gz / Format: CCP4 / Size: 30.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.014 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
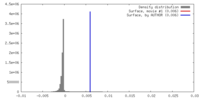
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Binary complex of DCL1 in complex with pri-miRNA 166f
| Entire | Name: Binary complex of DCL1 in complex with pri-miRNA 166f |
|---|---|
| Components |
|
-Supramolecule #1: Binary complex of DCL1 in complex with pri-miRNA 166f
| Supramolecule | Name: Binary complex of DCL1 in complex with pri-miRNA 166f / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Endoribonuclease Dicer homolog 1
| Macromolecule | Name: Endoribonuclease Dicer homolog 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO EC number: Hydrolases; Acting on ester bonds; Endoribonucleases producing 5'-phosphomonoesters |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 213.859344 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MVMEDEPREA TIKPSYWLDA CEDISCDLID DLVSEFDPSS VAVNESTDEN GVINDFFGGI DHILDSIKNG GGLPNNGVSD TNSQINEVT VTPQVIAKET VKENGLQKNG GKRDEFSKEE GDKDRKRARV CSYQSERSNL SGRGHVNNSR EGDRFMNRKR T RNWDEAGN ...String: MVMEDEPREA TIKPSYWLDA CEDISCDLID DLVSEFDPSS VAVNESTDEN GVINDFFGGI DHILDSIKNG GGLPNNGVSD TNSQINEVT VTPQVIAKET VKENGLQKNG GKRDEFSKEE GDKDRKRARV CSYQSERSNL SGRGHVNNSR EGDRFMNRKR T RNWDEAGN NKKKRECNNY RRDGRDREVR GYWERDKVGS NELVYRSGTW EADHERDVKK VSGGNRECDV KAEENKSKPE ER KEKVVEE QARRYQLDVL EQAKAKNTIA FLETGAGKTL IAILLIKSVH KDLMSQNRKM LSVFLVPKVP LVYQQAEVIR NQT CFQVGH YCGEMGQDFW DSRRWQREFE SKQVLVMTAQ ILLNILRHSI IRMETIDLLI LDECHHAVKK HPYSLVMSEF YHTT PKDKR PAIFGMTASP VNLKGVSSQV DCAIKIRNLE TKLDSTVCTI KDRKELEKHV PMPSEIVVEY DKAATMWSLH ETIKQ MIAA VEEAAQASSR KSKWQFMGAR DAGAKDELRQ VYGVSERTES DGAANLIHKL RAINYTLAEL GQWCAYKVGQ SFLSAL QSD ERVNFQVDVK FQESYLSEVV SLLQCELLEG AAAEKVAAEV GKPENGNAHD EMEEGELPDD PVVSGGEHVD EVIGAAV AD GKVTPKVQSL IKLLLKYQHT ADFRAIVFVE RVVAALVLPK VFAELPSLSF IRCASMIGHN NSQEMKSSQM QDTISKFR D GHVTLLVATS VAEEGLDIRQ CNVVMRFDLA KTVLAYIQSR GRARKPGSDY ILMVERGNVS HAAFLRNARN SEETLRKEA IERTDLSHLK DTSRLISIDA VPGTVYKVEA TGAMVSLNSA VGLVHFYCSQ LPGDRYAILR PEFSMEKHEK PGGHTEYSCR LQLPCNAPF EILEGPVCSS MRLAQQAVCL AACKKLHEMG AFTDMLLPDK GSGQDAEKAD QDDEGEPVPG TARHREFYPE G VADVLKGE WVSSGKEVCE SSKLFHLYMY NVRCVDFGSS KDPFLSEVSE FAILFGNELD AEVLSMSMDL YVARAMITKA SL AFKGSLD ITENQLSSLK KFHVRLMSIV LDVDVEPSTT PWDPAKAYLF VPVTDNTSME PIKGINWELV EKITKTTAWD NPL QRARPD VYLGTNERTL GGDRREYGFG KLRHNIVFGQ KSHPTYGIRG AVASFDVVRA SGLLPVRDAF EKEVEEDLSK GKLM MADGC MVAEDLIGKI VTAAHSGKRF YVDSICYDMS AETSFPRKEG YLGPLEYNTY ADYYKQKYGV DLNCKQQPLI KGRGV SYCK NLLSPRFEQS GESETVLDKT YYVFLPPELC VVHPLSGSLI RGAQRLPSIM RRVESMLLAV QLKNLISYPI PTSKIL EAL TAASCQETFC YERAELLGDA YLKWVVSRFL FLKYPQKHEG QLTRMRQQMV SNMVLYQFAL VKGLQSYIQA DRFAPSR WS APGVPPVFDE DTKDGGSSFF DEEQKPVSEE NSDVFEDGEM EDGELEGDLS SYRVLSSKTL ADVVEALIGV YYVEGGKI A ANHLMKWIGI HVEDDPDEVD GTLKNVNVPE SVLKSIDFVG LERALKYEFK EKGLLVEAIT HASRPSSGVS CYQRLEFVG DAVLDHLITR HLFFTYTSLP PGRLTDLRAA AVNNENFARV AVKHKLHLYL RHGSSALEKQ IREFVKEVQT ESSKPGFNSF GLGDCKAPK VLGDIVESIA GAIFLDSGKD TTAAWKVFQP LLQPMVTPET LPMHPVRELQ ERCQQQAEGL EYKASRSGNT A TVEVFIDG VQVGVAQNPQ KKMAQKLAAR NALAALKEKE IAESKEKHIN NGNAGEDQGE NENGNKKNGH QPFTRQTLND IC LRKNWPM PSYRCVKEGG PAHAKRFTFG VRVNTSDRGW TDECIGEPMP SVKKAKDSAA VLLLELLNKT FS UniProtKB: Endoribonuclease Dicer homolog 1 |
-Macromolecule #2: pri-miRNA 166f
| Macromolecule | Name: pri-miRNA 166f / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 47.200773 KDa |
| Sequence | String: GUCUUUCUGA GCCAAAAGUU CAGGUGAAUG AUGCCUGGCU CGAGACCAUU CAAUCUCAUG AUCUCAUGAU UAUAACGAUG AUGAUGAUG AUGUCGGACC AGGCUUCAUU CCCCUCAACU UACACGUUUU GCUUCUCAAU CUUCAAGAC GENBANK: GENBANK: AB016875.1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 64.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.6 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 653929 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)