+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Brain Glutamine Synthetase | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | human brain / Glutamine Synthetase / ligase | |||||||||
| Function / homology |  Function and homology information Function and homology informationintracellular ammonium homeostasis / protein palmitoylation / protein S-acyltransferase / Astrocytic Glutamate-Glutamine Uptake And Metabolism / protein-cysteine S-palmitoyltransferase activity / regulation of protein localization to nucleolus / regulation of sprouting angiogenesis / regulation of endothelial cell migration / glutamine synthetase / : ...intracellular ammonium homeostasis / protein palmitoylation / protein S-acyltransferase / Astrocytic Glutamate-Glutamine Uptake And Metabolism / protein-cysteine S-palmitoyltransferase activity / regulation of protein localization to nucleolus / regulation of sprouting angiogenesis / regulation of endothelial cell migration / glutamine synthetase / : / glutamine synthetase activity / Glutamate and glutamine metabolism / L-glutamate catabolic process / glial cell projection / response to glucose / positive regulation of erythrocyte differentiation / cellular response to starvation / ribosome biogenesis / cell body / angiogenesis / cell population proliferation / endoplasmic reticulum / mitochondrion / extracellular exosome / ATP binding / metal ion binding / identical protein binding / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.73 Å | |||||||||
 Authors Authors | Tringides ML | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Life Sci Alliance / Year: 2023 Journal: Life Sci Alliance / Year: 2023Title: A cryo-electron microscopic approach to elucidate protein structures from human brain microsomes. Authors: Marios L Tringides / Zhemin Zhang / Christopher E Morgan / Chih-Chia Su / Edward W Yu /  Abstract: We recently developed a "Build and Retrieve" cryo-electron microscopy (cryo-EM) methodology, which is capable of simultaneously producing near-atomic resolution cryo-EM maps for several individual ...We recently developed a "Build and Retrieve" cryo-electron microscopy (cryo-EM) methodology, which is capable of simultaneously producing near-atomic resolution cryo-EM maps for several individual proteins from a heterogeneous, multiprotein sample. Here we report the use of "Build and Retrieve" to define the composition of a raw human brain microsomal lysate. From this sample, we simultaneously identify and solve cryo-EM structures of five different brain enzymes whose functions affect neurotransmitter recycling, iron metabolism, glycolysis, axonal development, energy homeostasis, and retinoic acid biosynthesis. Interestingly, malfunction of these important proteins has been directly linked to several neurodegenerative disorders, such as Alzheimer's, Huntington's, and Parkinson's diseases. Our work underscores the importance of cryo-EM in facilitating tissue and organ proteomics at the atomic level. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27580.map.gz emd_27580.map.gz | 8.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27580-v30.xml emd-27580-v30.xml emd-27580.xml emd-27580.xml | 16.1 KB 16.1 KB | Display Display |  EMDB header EMDB header |
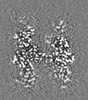
| Images |  emd_27580.png emd_27580.png | 645.1 KB | ||
| Filedesc metadata |  emd-27580.cif.gz emd-27580.cif.gz | 5.7 KB | ||
| Others |  emd_27580_half_map_1.map.gz emd_27580_half_map_1.map.gz emd_27580_half_map_2.map.gz emd_27580_half_map_2.map.gz | 151.5 MB 151.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27580 http://ftp.pdbj.org/pub/emdb/structures/EMD-27580 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27580 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27580 | HTTPS FTP |
-Related structure data
| Related structure data |  8dnuMC  8dnmC  8dnoC  8dnpC  8dnsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27580.map.gz / Format: CCP4 / Size: 9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27580.map.gz / Format: CCP4 / Size: 9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
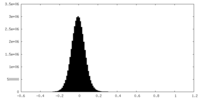
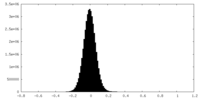
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_27580_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
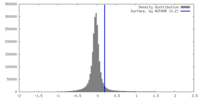
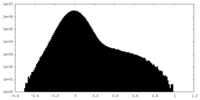
| Density Histograms |
-Half map: #1
| File | emd_27580_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
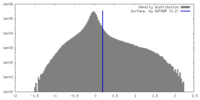
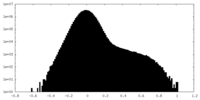
| Density Histograms |
- Sample components
Sample components
-Entire : Glutamine Synthetase
| Entire | Name: Glutamine Synthetase |
|---|---|
| Components |
|
-Supramolecule #1: Glutamine Synthetase
| Supramolecule | Name: Glutamine Synthetase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Glutamine synthetase
| Macromolecule | Name: Glutamine synthetase / type: protein_or_peptide / ID: 1 / Number of copies: 10 / Enantiomer: LEVO / EC number: glutamine synthetase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 42.118402 KDa |
| Sequence | String: MTTSASSHLN KGIKQVYMSL PQGEKVQAMY IWIDGTGEGL RCKTRTLDSE PKCVEELPEW NFDGSSTLQS EGSNSDMYLV PAAMFRDPF RKDPNKLVLC EVFKYNRRPA ETNLRHTCKR IMDMVSNQHP WFGMEQEYTL MGTDGHPFGW PSNGFPGPQG P YYCGVGAD ...String: MTTSASSHLN KGIKQVYMSL PQGEKVQAMY IWIDGTGEGL RCKTRTLDSE PKCVEELPEW NFDGSSTLQS EGSNSDMYLV PAAMFRDPF RKDPNKLVLC EVFKYNRRPA ETNLRHTCKR IMDMVSNQHP WFGMEQEYTL MGTDGHPFGW PSNGFPGPQG P YYCGVGAD RAYGRDIVEA HYRACLYAGV KIAGTNAEVM PAQWEFQIGP CEGISMGDHL WVARFILHRV CEDFGVIATF DP KPIPGNW NGAGCHTNFS TKAMREENGL KYIEEAIEKL SKRHQYHIRA YDPKGGLDNA RRLTGFHETS NINDFSAGVA NRS ASIRIP RTVGQEKKGY FEDRRPSANC DPFSVTEALI RTCLLNETGD EPFQYKN UniProtKB: Glutamine synthetase |
-Macromolecule #2: MANGANESE (II) ION
| Macromolecule | Name: MANGANESE (II) ION / type: ligand / ID: 2 / Number of copies: 10 / Formula: MN |
|---|---|
| Molecular weight | Theoretical: 54.938 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | tissue |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 35.3 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6580000000000001 µm / Nominal defocus min: 0.216 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)