[English] 日本語
 Yorodumi
Yorodumi- EMDB-26320: Cryo-EM structure of the pancreatic ATP-sensitive potassium chann... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the pancreatic ATP-sensitive potassium channel in the apo form with Kir6.2-CTD in the down conformation | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | KATP channel / SUR1 / Kir6.2 / ATP / sulfonylurea receptor / potassium transport / metabolic sensor / trafficking defects / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationRegulation of insulin secretion / ATP sensitive Potassium channels / ABC-family proteins mediated transport / response to resveratrol / ATP-activated inward rectifier potassium channel activity / inward rectifying potassium channel / sulfonylurea receptor activity / cell body fiber / ventricular cardiac muscle tissue development / CAMKK-AMPK signaling cascade ...Regulation of insulin secretion / ATP sensitive Potassium channels / ABC-family proteins mediated transport / response to resveratrol / ATP-activated inward rectifier potassium channel activity / inward rectifying potassium channel / sulfonylurea receptor activity / cell body fiber / ventricular cardiac muscle tissue development / CAMKK-AMPK signaling cascade / voltage-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / ATPase-coupled monoatomic cation transmembrane transporter activity / inward rectifier potassium channel activity / regulation of monoatomic ion transmembrane transport / nervous system process / inorganic cation transmembrane transport / response to stress / ankyrin binding / Ion homeostasis / response to ATP / action potential / potassium ion import across plasma membrane / response to testosterone / voltage-gated potassium channel activity / intercalated disc / potassium channel activity / axolemma / ABC-type transporter activity / negative regulation of insulin secretion / cellular response to nutrient levels / heat shock protein binding / T-tubule / potassium ion transmembrane transport / regulation of insulin secretion / acrosomal vesicle / regulation of membrane potential / determination of adult lifespan / response to ischemia / positive regulation of protein localization to plasma membrane / cellular response to glucose stimulus / potassium ion transport / sarcolemma / cellular response to nicotine / glucose metabolic process / cellular response to tumor necrosis factor / nuclear envelope / response to estradiol / presynaptic membrane / transmembrane transporter binding / response to hypoxia / endosome / response to xenobiotic stimulus / neuronal cell body / glutamatergic synapse / apoptotic process / ATP hydrolysis activity / protein-containing complex / ATP binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Cricetus cricetus (black-bellied hamster) / Cricetus cricetus (black-bellied hamster) /  | |||||||||
| Method | single particle reconstruction / cryo EM | |||||||||
 Authors Authors | Shyng SL / Sung MW / Driggers CM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2019 Journal: Elife / Year: 2019Title: Mechanism of pharmacochaperoning in a mammalian K channel revealed by cryo-EM. Authors: Gregory M Martin / Min Woo Sung / Zhongying Yang / Laura M Innes / Balamurugan Kandasamy / Larry L David / Craig Yoshioka / Show-Ling Shyng /  Abstract: ATP-sensitive potassium (K) channels composed of a pore-forming Kir6.2 potassium channel and a regulatory ABC transporter sulfonylurea receptor 1 (SUR1) regulate insulin secretion in pancreatic β- ...ATP-sensitive potassium (K) channels composed of a pore-forming Kir6.2 potassium channel and a regulatory ABC transporter sulfonylurea receptor 1 (SUR1) regulate insulin secretion in pancreatic β-cells to maintain glucose homeostasis. Mutations that impair channel folding or assembly prevent cell surface expression and cause congenital hyperinsulinism. Structurally diverse K inhibitors are known to act as pharmacochaperones to correct mutant channel expression, but the mechanism is unknown. Here, we compare cryoEM structures of a mammalian K channel bound to pharmacochaperones glibenclamide, repaglinide, and carbamazepine. We found all three drugs bind within a common pocket in SUR1. Further, we found the N-terminus of Kir6.2 inserted within the central cavity of the SUR1 ABC core, adjacent the drug binding pocket. The findings reveal a common mechanism by which diverse compounds stabilize the Kir6.2 N-terminus within SUR1's ABC core, allowing it to act as a firm 'handle' for the assembly of metastable mutant SUR1-Kir6.2 complexes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26320.map.gz emd_26320.map.gz | 1.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26320-v30.xml emd-26320-v30.xml emd-26320.xml emd-26320.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
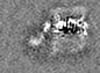
| Images |  emd_26320.png emd_26320.png | 113.5 KB | ||
| Filedesc metadata |  emd-26320.cif.gz emd-26320.cif.gz | 4.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26320 http://ftp.pdbj.org/pub/emdb/structures/EMD-26320 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26320 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26320 | HTTPS FTP |
-Validation report
| Summary document |  emd_26320_validation.pdf.gz emd_26320_validation.pdf.gz | 457.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26320_full_validation.pdf.gz emd_26320_full_validation.pdf.gz | 456.9 KB | Display | |
| Data in XML |  emd_26320_validation.xml.gz emd_26320_validation.xml.gz | 4.5 KB | Display | |
| Data in CIF |  emd_26320_validation.cif.gz emd_26320_validation.cif.gz | 4.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26320 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26320 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26320 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26320 | HTTPS FTP |
-Related structure data
| Related structure data |  7uqrMC  7tysC  7tytC  7u1eC  7u1qC  7u1sC  7u24C  7u2xC  7u6yC  7u7mC  7uaaC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26320.map.gz / Format: CCP4 / Size: 2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26320.map.gz / Format: CCP4 / Size: 2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
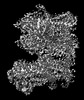
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.826 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : KATP-ATP-CTDup
| Entire | Name: KATP-ATP-CTDup |
|---|---|
| Components |
|
-Supramolecule #1: KATP-ATP-CTDup
| Supramolecule | Name: KATP-ATP-CTDup / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Cricetus cricetus (black-bellied hamster) Cricetus cricetus (black-bellied hamster) |
-Supramolecule #2: Kir6.2
| Supramolecule | Name: Kir6.2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 / Details: Adenovirus-based infection of INS-1 cells |
|---|---|
| Source (natural) | Organism:  Cricetus cricetus (black-bellied hamster) Cricetus cricetus (black-bellied hamster) |
-Supramolecule #3: SUR1
| Supramolecule | Name: SUR1 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 / Details: Adenovirus-based infection of INS-1 cells |
|---|---|
| Source (natural) | Organism:  |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.6 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Initial angle assignment | Type: COMMON LINE |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
-Atomic model buiding 1
| Initial model |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Output model |  PDB-7uqr: |
 Movie
Movie Controller
Controller
















 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)