+Search query
-Structure paper
| Title | Ligand-mediated Structural Dynamics of a Mammalian Pancreatic K Channel. |
|---|---|
| Journal, issue, pages | J Mol Biol, Vol. 434, Issue 19, Page 167789, Year 2022 |
| Publish date | Oct 15, 2022 |
 Authors Authors | Min Woo Sung / Camden M Driggers / Barmak Mostofian / John D Russo / Bruce L Patton / Daniel M Zuckerman / Show-Ling Shyng /  |
| PubMed Abstract | Regulation of pancreatic K channels involves orchestrated interactions of their subunits, Kir6.2 and SUR1, and ligands. Previously we reported K channel cryo-EM structures in the presence and absence ...Regulation of pancreatic K channels involves orchestrated interactions of their subunits, Kir6.2 and SUR1, and ligands. Previously we reported K channel cryo-EM structures in the presence and absence of pharmacological inhibitors and ATP, focusing on the mechanisms by which inhibitors act as pharmacological chaperones of K channels (Martin et al., 2019). Here we analyzed the same cryo-EM datasets with a focus on channel conformational dynamics to elucidate structural correlates pertinent to ligand interactions and channel gating. We found pharmacological inhibitors and ATP enrich a channel conformation in which the Kir6.2 cytoplasmic domain is closely associated with the transmembrane domain, while depleting one where the Kir6.2 cytoplasmic domain is extended away into the cytoplasm. This conformational change remodels a network of intra- and inter-subunit interactions as well as the ATP and PIP binding pockets. The structures resolved key contacts between the distal N-terminus of Kir6.2 and SUR1's ABC module involving residues implicated in channel function and showed a SUR1 residue, K134, participates in PIP binding. Molecular dynamics simulations revealed two Kir6.2 residues, K39 and R54, that mediate both ATP and PIP binding, suggesting a mechanism for competitive gating by ATP and PIP. |
 External links External links |  J Mol Biol / J Mol Biol /  PubMed:35964676 / PubMed:35964676 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.41 - 7.4 Å |
| Structure data | EMDB-26193, PDB-7tys: EMDB-26194, PDB-7tyt: EMDB-26299, PDB-7u1e: EMDB-26303, PDB-7u1q: EMDB-26304, PDB-7u1s: EMDB-26307, PDB-7u24: EMDB-26308: Cryo-EM structure of the pancreatic ATP-sensitive potassium channel bound to ATP and glibenclamide with Kir6.2-CTD in the down conformation EMDB-26309: Cryo-EM structure of the pancreatic ATP-sensitive potassium channel bound to ATP and in the presence of carbamazepine with Kir6.2-CTD in the up conformation EMDB-26312: Cryo-EM structure of the pancreatic ATP-sensitive potassium channel bound to ATP with Kir6.2-CTD in the up conformation EMDB-26320, PDB-7uqr: EMDB-26321, PDB-7u2x: |
| Chemicals |  ChemComp-ATP:  ChemComp-K:  ChemComp-POV:  ChemComp-P5S: 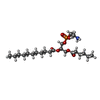 ChemComp-65I: 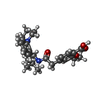 ChemComp-BJX:  ChemComp-PTY:  ChemComp-NAG: 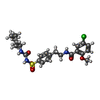 ChemComp-GBM: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / KATP channel / SUR1 / Kir6.2 / repaglinide / RPG / sulfonylurea receptor / potassium transport / glibenclamide / GBC / GBM / metabolic sensor |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers
























 cricetus cricetus (black-bellied hamster)
cricetus cricetus (black-bellied hamster)