[English] 日本語
 Yorodumi
Yorodumi- EMDB-25537: Structure of the wt IRES and 40S ribosome ternary complex, open c... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
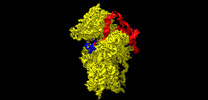
| Title | Structure of the wt IRES and 40S ribosome ternary complex, open conformation. Structure 11(wt) | |||||||||||||||
 Map data Map data | Post processed map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | HCV / IRES / 40S / RIBOSOME | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationmulti-eIF complex / translation factor activity, RNA binding / eukaryotic 43S preinitiation complex / ribosomal subunit / eukaryotic 48S preinitiation complex / Formation of the ternary complex, and subsequently, the 43S complex / laminin receptor activity / Ribosomal scanning and start codon recognition / Translation initiation complex formation / mammalian oogenesis stage ...multi-eIF complex / translation factor activity, RNA binding / eukaryotic 43S preinitiation complex / ribosomal subunit / eukaryotic 48S preinitiation complex / Formation of the ternary complex, and subsequently, the 43S complex / laminin receptor activity / Ribosomal scanning and start codon recognition / Translation initiation complex formation / mammalian oogenesis stage / activation-induced cell death of T cells / positive regulation of signal transduction by p53 class mediator / Formation of a pool of free 40S subunits / ubiquitin ligase inhibitor activity / phagocytic cup / GTP hydrolysis and joining of the 60S ribosomal subunit / 90S preribosome / L13a-mediated translational silencing of Ceruloplasmin expression / TOR signaling / endonucleolytic cleavage to generate mature 3'-end of SSU-rRNA from (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / T cell proliferation involved in immune response / erythrocyte development / ribosomal small subunit export from nucleus / translation regulator activity / laminin binding / rough endoplasmic reticulum / endonucleolytic cleavage in ITS1 to separate SSU-rRNA from 5.8S rRNA and LSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / gastrulation / MDM2/MDM4 family protein binding / cytosolic ribosome / translation initiation factor activity / class I DNA-(apurinic or apyrimidinic site) endonuclease activity / DNA-(apurinic or apyrimidinic site) lyase / ribosome assembly / rescue of stalled ribosome / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / maturation of SSU-rRNA / cellular response to leukemia inhibitory factor / small-subunit processome / translational initiation / protein kinase C binding / positive regulation of apoptotic signaling pathway / positive regulation of protein-containing complex assembly / placenta development / spindle / cytoplasmic ribonucleoprotein granule / modification-dependent protein catabolic process / G1/S transition of mitotic cell cycle / protein tag activity / rRNA processing / ribosomal small subunit biogenesis / rhythmic process / positive regulation of canonical Wnt signaling pathway / small ribosomal subunit rRNA binding / ribosome binding / glucose homeostasis / regulation of translation / ribosomal small subunit assembly / virus receptor activity / small ribosomal subunit / T cell differentiation in thymus / cytosolic small ribosomal subunit / cell body / cytoplasmic translation / perikaryon / tRNA binding / mitochondrial inner membrane / postsynaptic density / cell differentiation / rRNA binding / ribosome / protein ubiquitination / structural constituent of ribosome / positive regulation of apoptotic process / ribonucleoprotein complex / positive regulation of protein phosphorylation / translation / cell division / DNA repair / mRNA binding / centrosome / positive regulation of cell population proliferation / ubiquitin protein ligase binding / dendrite / synapse / negative regulation of apoptotic process / nucleolus / apoptotic process / protein kinase binding / perinuclear region of cytoplasm / Golgi apparatus / endoplasmic reticulum / DNA binding / RNA binding / zinc ion binding / membrane / nucleus / metal ion binding / plasma membrane / cytosol Similarity search - Function | |||||||||||||||
| Biological species |   Hepatitis C virus (isolate 1) / Hepatitis C virus (isolate 1) /  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||||||||
 Authors Authors | Brown ZP / Abaeva IS / De S / Hellen CUT / Pestova TV / Frank J | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: EMBO J / Year: 2022 Journal: EMBO J / Year: 2022Title: Molecular architecture of 40S translation initiation complexes on the hepatitis C virus IRES. Authors: Zuben P Brown / Irina S Abaeva / Swastik De / Christopher U T Hellen / Tatyana V Pestova / Joachim Frank /  Abstract: Hepatitis C virus mRNA contains an internal ribosome entry site (IRES) that mediates end-independent translation initiation, requiring a subset of eukaryotic initiation factors (eIFs). Biochemical ...Hepatitis C virus mRNA contains an internal ribosome entry site (IRES) that mediates end-independent translation initiation, requiring a subset of eukaryotic initiation factors (eIFs). Biochemical studies revealed that direct binding of the IRES to the 40S ribosomal subunit places the initiation codon into the P site, where it base pairs with eIF2-bound Met-tRNAiMet forming a 48S initiation complex. Subsequently, eIF5 and eIF5B mediate subunit joining, yielding an elongation-competent 80S ribosome. Initiation can also proceed without eIF2, in which case Met-tRNAiMet is recruited directly by eIF5B. However, the structures of initiation complexes assembled on the HCV IRES, the transitions between different states, and the accompanying conformational changes have remained unknown. To fill these gaps, we now obtained cryo-EM structures of IRES initiation complexes, at resolutions up to 3.5 Å, that cover all major stages from the initial ribosomal association, through eIF2-containing 48S initiation complexes, to eIF5B-containing complexes immediately prior to subunit joining. These structures provide insights into the dynamic network of 40S/IRES contacts, highlight the role of IRES domain II, and reveal conformational changes that occur during the transition from eIF2- to eIF5B-containing 48S complexes and prepare them for subunit joining. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25537.map.gz emd_25537.map.gz | 228.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25537-v30.xml emd-25537-v30.xml emd-25537.xml emd-25537.xml | 60.7 KB 60.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_25537_fsc.xml emd_25537_fsc.xml | 14.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_25537.png emd_25537.png | 36.6 KB | ||
| Masks |  emd_25537_msk_1.map emd_25537_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-25537.cif.gz emd-25537.cif.gz | 12.6 KB | ||
| Others |  emd_25537_additional_1.map.gz emd_25537_additional_1.map.gz emd_25537_additional_2.map.gz emd_25537_additional_2.map.gz emd_25537_additional_3.map.gz emd_25537_additional_3.map.gz emd_25537_half_map_1.map.gz emd_25537_half_map_1.map.gz emd_25537_half_map_2.map.gz emd_25537_half_map_2.map.gz | 122.1 MB 144.3 MB 190.7 MB 194.2 MB 194.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25537 http://ftp.pdbj.org/pub/emdb/structures/EMD-25537 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25537 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25537 | HTTPS FTP |
-Validation report
| Summary document |  emd_25537_validation.pdf.gz emd_25537_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25537_full_validation.pdf.gz emd_25537_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_25537_validation.xml.gz emd_25537_validation.xml.gz | 20.9 KB | Display | |
| Data in CIF |  emd_25537_validation.cif.gz emd_25537_validation.cif.gz | 27.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25537 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25537 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25537 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25537 | HTTPS FTP |
-Related structure data
| Related structure data |  7syqMC  7syiC  7syjC  7sykC  7sylC  7syoC  7sypC  7syrC  7sysC  7sytC  7syuC  7syvC  7sywC  7syxC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25537.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25537.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Post processed map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.95 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_25537_msk_1.map emd_25537_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Local resolution values
| File | emd_25537_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution values | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Local resolution map filtered at local resolution
| File | emd_25537_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution map filtered at local resolution | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map
| File | emd_25537_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map
| File | emd_25537_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map
| File | emd_25537_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : 40S ribosomal small subunit with HCV IRES
+Supramolecule #1: 40S ribosomal small subunit with HCV IRES
+Macromolecule #1: 18S rRNA
+Macromolecule #37: HCV IRES
+Macromolecule #2: Eukaryotic translation initiation factor 1A, X-chromosomal
+Macromolecule #3: uS2 (SA)
+Macromolecule #4: eS1
+Macromolecule #5: uS5
+Macromolecule #6: uS3
+Macromolecule #7: eS4 (S4 X isoform)
+Macromolecule #8: uS7
+Macromolecule #9: eS6
+Macromolecule #10: eS7
+Macromolecule #11: eS8
+Macromolecule #12: uS4
+Macromolecule #13: eS10
+Macromolecule #14: uS17
+Macromolecule #15: eS12
+Macromolecule #16: uS15
+Macromolecule #17: uS11
+Macromolecule #18: uS19
+Macromolecule #19: uS9
+Macromolecule #20: eS17
+Macromolecule #21: uS13
+Macromolecule #22: eS19
+Macromolecule #23: uS10
+Macromolecule #24: eS21
+Macromolecule #25: uS8
+Macromolecule #26: uS12
+Macromolecule #27: eS24
+Macromolecule #28: eS25
+Macromolecule #29: eS26
+Macromolecule #30: eS27
+Macromolecule #31: eS28
+Macromolecule #32: uS14
+Macromolecule #33: eS30
+Macromolecule #34: eS31
+Macromolecule #35: Receptor for Activated C Kinase 1 (RACK1)
+Macromolecule #36: eL41
+Macromolecule #38: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.000075 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R0.6/1 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Support film - Film thickness: 50 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 25 sec. / Pretreatment - Atmosphere: OTHER / Details: H2/O2 mixture for 25 seconds at 25W power |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: 4 second blot time, force 3. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI F30 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average exposure time: 4.0 sec. / Average electron dose: 70.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.26 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 56000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai F30 / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)