[English] 日本語
 Yorodumi
Yorodumi- EMDB-23124: Cryo-electron microcospy reconstruction of CH848.3.D0949.10.17chi... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23124 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-electron microcospy reconstruction of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env | |||||||||
 Map data Map data | Final sharpened map of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / host cell plasma membrane ...positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
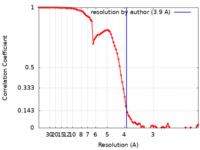
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Edwards RJ / Acharya P | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2021 Journal: Cell / Year: 2021Title: Fab-dimerized glycan-reactive antibodies are a structural category of natural antibodies. Authors: Wilton B Williams / R Ryan Meyerhoff / R J Edwards / Hui Li / Kartik Manne / Nathan I Nicely / Rory Henderson / Ye Zhou / Katarzyna Janowska / Katayoun Mansouri / Sophie Gobeil / Tyler ...Authors: Wilton B Williams / R Ryan Meyerhoff / R J Edwards / Hui Li / Kartik Manne / Nathan I Nicely / Rory Henderson / Ye Zhou / Katarzyna Janowska / Katayoun Mansouri / Sophie Gobeil / Tyler Evangelous / Bhavna Hora / Madison Berry / A Yousef Abuahmad / Jordan Sprenz / Margaret Deyton / Victoria Stalls / Megan Kopp / Allen L Hsu / Mario J Borgnia / Guillaume B E Stewart-Jones / Matthew S Lee / Naomi Bronkema / M Anthony Moody / Kevin Wiehe / Todd Bradley / S Munir Alam / Robert J Parks / Andrew Foulger / Thomas Oguin / Gregory D Sempowski / Mattia Bonsignori / Celia C LaBranche / David C Montefiori / Michael Seaman / Sampa Santra / John Perfect / Joseph R Francica / Geoffrey M Lynn / Baptiste Aussedat / William E Walkowicz / Richard Laga / Garnett Kelsoe / Kevin O Saunders / Daniela Fera / Peter D Kwong / Robert A Seder / Alberto Bartesaghi / George M Shaw / Priyamvada Acharya / Barton F Haynes /   Abstract: Natural antibodies (Abs) can target host glycans on the surface of pathogens. We studied the evolution of glycan-reactive B cells of rhesus macaques and humans using glycosylated HIV-1 envelope (Env) ...Natural antibodies (Abs) can target host glycans on the surface of pathogens. We studied the evolution of glycan-reactive B cells of rhesus macaques and humans using glycosylated HIV-1 envelope (Env) as a model antigen. 2G12 is a broadly neutralizing Ab (bnAb) that targets a conserved glycan patch on Env of geographically diverse HIV-1 strains using a unique heavy-chain (V) domain-swapped architecture that results in fragment antigen-binding (Fab) dimerization. Here, we describe HIV-1 Env Fab-dimerized glycan (FDG)-reactive bnAbs without V-swapped domains from simian-human immunodeficiency virus (SHIV)-infected macaques. FDG Abs also recognized cell-surface glycans on diverse pathogens, including yeast and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike. FDG precursors were expanded by glycan-bearing immunogens in macaques and were abundant in HIV-1-naive humans. Moreover, FDG precursors were predominately mutated IgMIgDCD27, thus suggesting that they originated from a pool of antigen-experienced IgM or marginal zone B cells. #1:  Journal: bioRxiv / Year: 2020 Journal: bioRxiv / Year: 2020Title: Fab-dimerized glycan-reactive antibodies neutralize HIV and are prevalent in humans and rhesus macaques Authors: Williams WB / Meyerhoff RR / Edwards RJ / Li H / Nicely NI / Henderson R / Zhou Y / Janowska K / Mansouri K / Manne K / Stalls V / Hsu AL / Borgnia MJ / Stewart-Jones G / Lee MS / Bronkema N ...Authors: Williams WB / Meyerhoff RR / Edwards RJ / Li H / Nicely NI / Henderson R / Zhou Y / Janowska K / Mansouri K / Manne K / Stalls V / Hsu AL / Borgnia MJ / Stewart-Jones G / Lee MS / Bronkema N / Perfect J / Moody MA / Wiehe K / Bradley T / Kepler TB / Alam SM / Foulger A / Bonsignori M / LaBranche CC / Montefiori DC / Seaman M / Santra S / Francica JR / Lynn GM / Aussedat B / Walkowicz WE / Laga R / Kelso G / Saunders KO / Fera D / Kwong PD / Seder RA / Bartesaghi A / Shaw GM / Acharya P / Haynes BF | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23124.map.gz emd_23124.map.gz | 4.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23124-v30.xml emd-23124-v30.xml emd-23124.xml emd-23124.xml | 30.3 KB 30.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_23124_fsc.xml emd_23124_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_23124.png emd_23124.png | 204.5 KB | ||
| Masks |  emd_23124_msk_1.map emd_23124_msk_1.map emd_23124_msk_2.map emd_23124_msk_2.map | 64 MB 64 MB |  Mask map Mask map | |
| Others |  emd_23124_additional_1.map.gz emd_23124_additional_1.map.gz emd_23124_additional_2.map.gz emd_23124_additional_2.map.gz emd_23124_half_map_1.map.gz emd_23124_half_map_1.map.gz emd_23124_half_map_2.map.gz emd_23124_half_map_2.map.gz | 31.8 MB 4.6 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23124 http://ftp.pdbj.org/pub/emdb/structures/EMD-23124 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23124 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23124 | HTTPS FTP |
-Validation report
| Summary document |  emd_23124_validation.pdf.gz emd_23124_validation.pdf.gz | 503.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23124_full_validation.pdf.gz emd_23124_full_validation.pdf.gz | 502.7 KB | Display | |
| Data in XML |  emd_23124_validation.xml.gz emd_23124_validation.xml.gz | 16.2 KB | Display | |
| Data in CIF |  emd_23124_validation.cif.gz emd_23124_validation.cif.gz | 21 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23124 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23124 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23124 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23124 | HTTPS FTP |
-Related structure data
| Related structure data |  7l6oMC  6vtuC  6xrjC  7l02C  7l06C  7l09C  7l6mC  7lu9C  7luaC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23124.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23124.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Final sharpened map of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0665 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23124_msk_1.map emd_23124_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Mask #2
| File |  emd_23124_msk_2.map emd_23124_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env
| File | emd_23124_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Local resolution of of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env...
| File | emd_23124_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Local resolution of of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map A of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env
| File | emd_23124_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map A of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map B of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env
| File | emd_23124_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map B of CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 HIV Env | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : HIV Env CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 unliganded trimer...
| Entire | Name: HIV Env CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 unliganded trimer in the presence of but not bound to DH898.1 Fab |
|---|---|
| Components |
|
-Supramolecule #1: HIV Env CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 unliganded trimer...
| Supramolecule | Name: HIV Env CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 unliganded trimer in the presence of but not bound to DH898.1 Fab type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 / Strain: CH848 10.17 Human immunodeficiency virus 1 / Strain: CH848 10.17 |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: 293F Homo sapiens (human) / Recombinant cell: 293F |
| Molecular weight | Theoretical: 209.7 KDa |
-Macromolecule #1: CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 - gp120
| Macromolecule | Name: CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 - gp120 / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AENLWVTVYY GVPVWKEAKT TLFCASDARA YEKEVHNVWA THACVPTDPS PQELVLGNVT ENFNMWKNDM VDQMHEDIIS LWDQSLKPC VKLTPLCVTL ICSNATVKNG TVEEMKNCSF NTTTEIRDKE KKEYALFYKP DIVPLSETNN TSEYRLINCN T SACTQACP ...String: AENLWVTVYY GVPVWKEAKT TLFCASDARA YEKEVHNVWA THACVPTDPS PQELVLGNVT ENFNMWKNDM VDQMHEDIIS LWDQSLKPC VKLTPLCVTL ICSNATVKNG TVEEMKNCSF NTTTEIRDKE KKEYALFYKP DIVPLSETNN TSEYRLINCN T SACTQACP KVTFEPIPIH YCAPAGYAIL KCNDETFNGT GPCSNVSTVQ CTHGIRPVVS TQLLLNGSLA EKEIVIRSEN LT NNAKIII VHLHTPVEIV CTRPNNNTRK SVRIGPGQTF YATGDIIGDI KQAHCNISEE KWNDTLQKVG IELQKHFPNK TIK YNQSAG GDMEITTHSF NCGGEFFYCN TSNLFNGTYN GTYISTNSSA NSTSTITLQC RIKQIINMWQ GVGRCMYAPP IAGN ITCRS NITGLLLTRD GGTNSNETET FRPAGGDMRD NWRSELYKYK VVKIEPLGVA PTRCKRRVVG R |
-Macromolecule #2: CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 - gp41
| Macromolecule | Name: CH848.3.D0949.10.17chim.6R.DS.SOSIP.664 - gp41 / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Sequence | String: FLGFLGAAGS TMGAASMTLT VQARNLLSGI VQQQSNLLRA PEAQQHLLKL TVWGIKQLQA RVLAVERYLR DQQLLGIWGC SGKLICCTN VPWNSSWSNR NLSEIWDNMT WLQWDKEISN YTQIIYGLLE ESQNQQEKNE QDLLALD |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: C-flat-1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 12.0 nm / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Pressure: 0.039 kPa / Details: Glow discharged for 30 s in PELCO easiGlow(TM). | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 293 K / Instrument: LEICA EM GP Details: 2.5 microliter of smaple applied to grid and incubated for 30 seconds in chamber, blotted for 2.5 seconds, and plunge frozen in liquid ethane. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 93.15 K / Max: 93.15 K |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 3230 / Average exposure time: 4.0 sec. / Average electron dose: 62.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)