+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22811 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Full-length human mitochondrial Hsp90 (TRAP1) with AMP-PNP | |||||||||||||||||||||
 Map data Map data | ||||||||||||||||||||||
 Sample Sample |
| |||||||||||||||||||||
 Keywords Keywords | Hsp90 / CHAPERONE / TRAP1 | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationtranslational attenuation / negative regulation of cellular respiration / Citric acid cycle (TCA cycle) / Respiratory electron transport / tumor necrosis factor receptor binding / negative regulation of intrinsic apoptotic signaling pathway in response to hydrogen peroxide / negative regulation of reactive oxygen species biosynthetic process / : / ATP-dependent protein folding chaperone / mitochondrial intermembrane space ...translational attenuation / negative regulation of cellular respiration / Citric acid cycle (TCA cycle) / Respiratory electron transport / tumor necrosis factor receptor binding / negative regulation of intrinsic apoptotic signaling pathway in response to hydrogen peroxide / negative regulation of reactive oxygen species biosynthetic process / : / ATP-dependent protein folding chaperone / mitochondrial intermembrane space / unfolded protein binding / protein folding / mitochondrial inner membrane / mitochondrial matrix / protein kinase binding / ATP hydrolysis activity / mitochondrion / RNA binding / nucleoplasm / ATP binding / membrane Similarity search - Function | |||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||
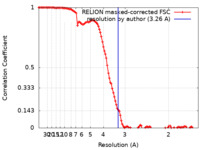
| Method | single particle reconstruction / cryo EM / Resolution: 3.26 Å | |||||||||||||||||||||
 Authors Authors | Liu YX / Agard DA | |||||||||||||||||||||
| Funding support |  United States, 6 items United States, 6 items
| |||||||||||||||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Cryo-EM reveals the dynamic interplay between mitochondrial Hsp90 and SdhB folding intermediates Authors: Liu YX / Agard DA / Elnatan D / Sun M / Myasnikov AG | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22811.map.gz emd_22811.map.gz | 116.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22811-v30.xml emd-22811-v30.xml emd-22811.xml emd-22811.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_22811_fsc.xml emd_22811_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_22811.png emd_22811.png | 108.4 KB | ||
| Masks |  emd_22811_msk_1.map emd_22811_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-22811.cif.gz emd-22811.cif.gz | 6 KB | ||
| Others |  emd_22811_half_map_1.map.gz emd_22811_half_map_1.map.gz emd_22811_half_map_2.map.gz emd_22811_half_map_2.map.gz | 98.4 MB 98.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22811 http://ftp.pdbj.org/pub/emdb/structures/EMD-22811 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22811 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22811 | HTTPS FTP |
-Validation report
| Summary document |  emd_22811_validation.pdf.gz emd_22811_validation.pdf.gz | 908 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22811_full_validation.pdf.gz emd_22811_full_validation.pdf.gz | 907.6 KB | Display | |
| Data in XML |  emd_22811_validation.xml.gz emd_22811_validation.xml.gz | 18.7 KB | Display | |
| Data in CIF |  emd_22811_validation.cif.gz emd_22811_validation.cif.gz | 24.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22811 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22811 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22811 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22811 | HTTPS FTP |
-Related structure data
| Related structure data |  7kckMC  7kclC  7kcmC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22811.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22811.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.814 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_22811_msk_1.map emd_22811_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_22811_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_22811_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human Trap1 dimer with AMP-PNP
| Entire | Name: human Trap1 dimer with AMP-PNP |
|---|---|
| Components |
|
-Supramolecule #1: human Trap1 dimer with AMP-PNP
| Supramolecule | Name: human Trap1 dimer with AMP-PNP / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 147 KDa |
-Macromolecule #1: Heat shock protein 75 kDa, mitochondrial
| Macromolecule | Name: Heat shock protein 75 kDa, mitochondrial / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 74.17757 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GIDPFTSTQT AEDKEEPLHS IISSTESVQG STSKHEFQAE TKKLLDIVAR SLYSEKEVFI RELISNASDA LEKLRHKLVS DGQALPEME IHLQTNAEKG TITIQDTGIG MTQEELVSNL GTIARSGSKA FLDALQNQAE ASSKIIGQFG VGFYSAFMVA D RVEVYSRS ...String: GIDPFTSTQT AEDKEEPLHS IISSTESVQG STSKHEFQAE TKKLLDIVAR SLYSEKEVFI RELISNASDA LEKLRHKLVS DGQALPEME IHLQTNAEKG TITIQDTGIG MTQEELVSNL GTIARSGSKA FLDALQNQAE ASSKIIGQFG VGFYSAFMVA D RVEVYSRS AAPGSLGYQW LSDGSGVFEI AEASGVRTGT KIIIHLKSDC KEFSSEARVR DVVTKYSNFV SFPLYLNGRR MN TLQAIWM MDPKDVGEWQ HEEFYRYVAQ AHDKPRYTLH YKTDAPLNIR SIFYVPDMKP SMFDVSRELG SSVALYSRKV LIQ TKATDI LPKWLRFIRG VVDSEDIPLN LSRELLQESA LIRKLRDVLQ QRLIKFFIDQ SKKDAEKYAK FFEDYGLFMR EGIV TATEQ EVKEDIAKLL RYESSALPSG QLTSLSEYAS RMRAGTRNIY YLCAPNRHLA EHSPYYEAMK KKDTEVLFCF EQFDE LTLL HLREFDKKKL ISVETDIVVD HYKEEKFEDR SPAAECLSEK ETEELMAWMR NVLGSRVTNV KVTLRLDTHP AMVTVL EMG AARHFLRMQQ LAKTQEERAQ LLQPTLEINP RHALIKKLNQ LRASEPGLAQ LLVDQIYENA MIAAGLVDDP RAMVGRL NE LLVKALERH UniProtKB: Heat shock protein 75 kDa, mitochondrial |
-Macromolecule #2: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 2 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #3: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 3 / Number of copies: 2 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Macromolecule #4: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOAMINOPHOSPHONIC ACID-ADENYLATE ESTER / type: ligand / ID: 4 / Number of copies: 2 / Formula: ANP |
|---|---|
| Molecular weight | Theoretical: 506.196 Da |
| Chemical component information |  ChemComp-ANP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 8 sec. |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 72.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT |
|---|---|
| Output model |  PDB-7kck: |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)