+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-20517 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
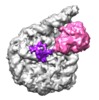
| Title | Set2 bound to nucleosome | |||||||||
 Map data Map data | Set2 bound to nucleosome, Class Ub | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Set2 / nucleosome / chromatin / KMT / GENE REGULATION | |||||||||
| Function / homology |  Function and homology information Function and homology information[histone H3]-lysine36 N-trimethyltransferase / histone H3K36 trimethyltransferase activity / ribosomal large subunit export from nucleus / modification-dependent protein catabolic process / protein tag activity / structural constituent of chromatin / heterochromatin formation / nucleosome / nucleosome assembly / ribosome biogenesis ...[histone H3]-lysine36 N-trimethyltransferase / histone H3K36 trimethyltransferase activity / ribosomal large subunit export from nucleus / modification-dependent protein catabolic process / protein tag activity / structural constituent of chromatin / heterochromatin formation / nucleosome / nucleosome assembly / ribosome biogenesis / chromosome / ribosomal large subunit assembly / methylation / cytosolic large ribosomal subunit / cytoplasmic translation / protein ubiquitination / structural constituent of ribosome / protein heterodimerization activity / ubiquitin protein ligase binding / regulation of DNA-templated transcription / mitochondrion / DNA binding / nucleus / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Chaetomium thermophilum (fungus) / synthetic construct (others) / Chaetomium thermophilum (fungus) / synthetic construct (others) /  Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) Chaetomium thermophilum (strain DSM 1495 / CBS 144.50 / IMI 039719) (fungus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||
 Authors Authors | Halic M / Bilokapic S | |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2019 Journal: Nat Commun / Year: 2019Title: Nucleosome and ubiquitin position Set2 to methylate H3K36. Authors: Silvija Bilokapic / Mario Halic /  Abstract: Histone H3 lysine 36 methylation (H3K36me) is a conserved histone modification deposited by the Set2 methyltransferases. Recent findings show that over-expression or mutation of Set2 enzymes promotes ...Histone H3 lysine 36 methylation (H3K36me) is a conserved histone modification deposited by the Set2 methyltransferases. Recent findings show that over-expression or mutation of Set2 enzymes promotes cancer progression, however, mechanisms of H3K36me are poorly understood. Set2 enzymes show spurious activity on histones and histone tails, and it is unknown how they obtain specificity to methylate H3K36 on the nucleosome. In this study, we present 3.8 Å cryo-EM structure of Set2 bound to the mimic of H2B ubiquitinated nucleosome. Our structure shows that Set2 makes extensive interactions with the H3 αN, the H3 tail, the H2A C-terminal tail and stabilizes DNA in the unwrapped conformation, which positions Set2 to specifically methylate H3K36. Moreover, we show that ubiquitin contributes to Set2 positioning on the nucleosome and stimulates the methyltransferase activity. Notably, our structure uncovers interfaces that can be targeted by small molecules for development of future cancer therapies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_20517.map.gz emd_20517.map.gz | 47.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-20517-v30.xml emd-20517-v30.xml emd-20517.xml emd-20517.xml | 23.8 KB 23.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_20517.png emd_20517.png | 424 KB | ||
| Filedesc metadata |  emd-20517.cif.gz emd-20517.cif.gz | 7.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-20517 http://ftp.pdbj.org/pub/emdb/structures/EMD-20517 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20517 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-20517 | HTTPS FTP |
-Related structure data
| Related structure data |  6px3MC  0559C  6nzoC  6px1C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_20517.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_20517.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Set2 bound to nucleosome, Class Ub | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Set2 bound to ubiquitinated nucleosome
+Supramolecule #1: Set2 bound to ubiquitinated nucleosome
+Supramolecule #2: Histone-lysine N-methyltransferase
+Supramolecule #3: DNA
+Supramolecule #4: Histone H3, Histone H4, Ubiquitin-60S ribosomal protein L40,Histo...
+Macromolecule #1: Histone H3
+Macromolecule #2: Histone H4
+Macromolecule #3: Ubiquitin-60S ribosomal protein L40,Histone H2A
+Macromolecule #4: Histone H2B 1.1
+Macromolecule #7: Histone-lysine N-methyltransferase
+Macromolecule #5: DNA (145-MER)
+Macromolecule #6: DNA (145-MER)
+Macromolecule #8: ZINC ION
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | #0 - Image recording ID: 1 / #0 - Film or detector model: GATAN K2 SUMMIT (4k x 4k) / #0 - Detector mode: COUNTING / #0 - Average electron dose: 75.0 e/Å2 / #1 - Image recording ID: 2 / #1 - Film or detector model: GATAN K2 BASE (4k x 4k) / #1 - Average electron dose: 75.0 e/Å2 / #2 - Image recording ID: 3 / #2 - Film or detector model: GATAN K2 BASE (4k x 4k) / #2 - Average electron dose: 75.0 e/Å2 / #3 - Image recording ID: 4 / #3 - Film or detector model: GATAN K2 BASE (4k x 4k) / #3 - Average electron dose: 75.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















